M-M-R II- measles, mumps, and rubella virus vaccine live injection, powder, lyophilized, for suspension
Merck Sharp & Dohme Corp.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use M-M-R II safely and effectively. See full prescribing information for M-M-R II.
M-M-R® II (Measles, Mumps, and Rubella Virus Vaccine Live) Suspension for subcutaneous injection Initial U.S. Approval: 1978 INDICATIONS AND USAGEM-M-R II is a vaccine indicated for active immunization for the prevention of measles, mumps, and rubella in individuals 12 months of age and older. (1) DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHSSuspension for injection (0.5-mL dose) supplied as a lyophilized vaccine to be reconstituted using accompanying sterile diluent. (3) CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSSee full prescribing information for adverse reactions occurring during clinical trials or the post-marketing period. (6)
DRUG INTERACTIONSUSE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 12/2020 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
M-M-R® II is a vaccine indicated for active immunization for the prevention of measles, mumps, and rubella in individuals 12 months of age and older.
2 DOSAGE AND ADMINISTRATION
For subcutaneous use only.
2.1 Dose and Schedule
Each 0.5 mL dose is administered subcutaneously.
The first dose is administered at 12 to 15 months of age. A second dose is administered at 4 to 6 years of age.
The second dose may be administered prior to 4 years of age, provided that there is a minimum interval of one month between the doses of measles, mumps and rubella virus vaccine, live {1-2}.
Children who received an initial dose of measles, mumps and rubella vaccine prior to their first birthday should receive additional doses of vaccine at 12-15 months of age and at 4-6 years of age to complete the vaccination series [see Clinical Studies (14.2)].
For post-exposure prophylaxis for measles, administer a dose of M-M-R II vaccine within 72 hours after exposure.
2.2 Preparation and Administration
Use a sterile syringe free of preservatives, antiseptics, and detergents for each injection and/or reconstitution of the vaccine because these substances may inactivate the live virus vaccine. To reconstitute, use only the diluent supplied with the vaccine since it is free of preservatives or other antiviral substances which might inactivate the vaccine.
Withdraw the entire volume of the supplied diluent from its vial and inject into lyophilized vaccine vial. Agitate to dissolve completely. Discard if the lyophilized vaccine cannot be dissolved.
Withdraw the entire volume of the reconstituted vaccine and inject subcutaneously into the outer aspect of the upper arm (deltoid region) or into the higher anterolateral area of the thigh.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Visually inspect the vaccine before and after reconstitution prior to administration. Before reconstitution, the lyophilized vaccine is a light yellow compact crystalline plug, when reconstituted, is a clear yellow liquid. Discard if particulate matter or discoloration are observed in the reconstituted vaccine.
To minimize loss of potency, administer M-M-R II as soon as possible after reconstitution. If not used immediately, the reconstituted vaccine may be stored between 36°F to 46°F (2°C to 8°C), protected from light, for up to 8 hours. Discard reconstituted vaccine if it is not used within 8 hours.
3 DOSAGE FORMS AND STRENGTHS
M-M-R II vaccine is a suspension for injection supplied as a single dose vial of lyophilized vaccine to be reconstituted using the accompanying sterile diluent [see Dosage and Administration (2.2) and How Supplied/Storage and Handling (16)]. A single dose after reconstitution is 0.5 mL.
4 CONTRAINDICATIONS
4.1 Hypersensitivity
Do not administer M-M-R II vaccine to individuals with a history of hypersensitivity to any component of the vaccine (including gelatin) {3} or who have experienced a hypersensitivity reaction following administration of a previous dose of M-M-R II vaccine or any other measles, mumps and rubella-containing vaccine. Do not administer M-M-R II vaccine to individuals with a history of anaphylaxis to neomycin [see Description (11)].
4.2 Immunosuppression
Do not administer M-M-R II vaccine to individuals who are immunodeficient or immunosuppressed due to disease or medical therapy. Measles inclusion body encephalitis {4} (MIBE), pneumonitis {5} and death as a direct consequence of disseminated measles vaccine virus infection have been reported in immunocompromised individuals inadvertently vaccinated with measles-containing vaccine. In this population, disseminated mumps and rubella vaccine virus infection have also been reported.
4.3 Moderate or Severe Febrile Illness
Do not administer M-M-R II vaccine to individuals with an active febrile illness with fever >101.3°F (>38.5°C).
4.4 Active Untreated Tuberculosis
Do not administer M-M-R II vaccine to individuals with active untreated tuberculosis (TB).
4.5 Pregnancy
Do not administer M-M-R II to individuals who are pregnant or who are planning on becoming pregnant within the next month [see Use in Specific Populations (8.1) and Patient Counseling Information (17)].
5 WARNINGS AND PRECAUTIONS
5.1 Febrile Seizure
There is a risk of fever and associated febrile seizure in the first 2 weeks following immunization with M-M-R II vaccine. For children who have experienced a previous febrile seizure (from any cause) and those with a family history of febrile seizures there is a small increase in risk of febrile seizure following receipt of M-M-R II vaccine [see Adverse Reactions (6)].
5.2 Hypersensitivity to Eggs
Individuals with a history of anaphylactic, anaphylactoid, or other immediate reactions (e.g., hives, swelling of the mouth and throat, difficulty breathing, hypotension, or shock) subsequent to egg ingestion may be at an enhanced risk of immediate-type hypersensitivity reactions after receiving M-M-R II vaccine. The potential risks and known benefits should be evaluated before considering vaccination in these individuals.
5.3 Thrombocytopenia
Transient thrombocytopenia has been reported within 4-6 weeks following vaccination with measles, mumps and rubella vaccine. Carefully evaluate the potential risk and benefit of vaccination in children with thrombocytopenia or in those who experienced thrombocytopenia after vaccination with a previous dose of measles, mumps, and rubella vaccine {6-8} [see Adverse Reactions (6)].
5.4 Family History of Immunodeficiency
Vaccination should be deferred in individuals with a family history of congenital or hereditary immunodeficiency until the individual’s immune status has been evaluated and the individual has been found to be immunocompetent.
5.5 Immune Globulins and Transfusions
Immune Globulins (IG) and other blood products should not be given concurrently with M-M-R II [see Drug Interactions (7.2)]. These products may contain antibodies that interfere with vaccine virus replication and decrease the expected immune response.
The Advisory Committee on Immunization Practices (ACIP) has specific recommendations for intervals between administration of antibody containing products and live virus vaccines.
6 ADVERSE REACTIONS
The following adverse reactions include those identified during clinical trials or reported during post-approval use of M-M-R II vaccine or its individual components.
Body as a Whole
Panniculitis; atypical measles; fever; syncope; headache; dizziness; malaise; irritability.
Cardiovascular System
Vasculitis.
Digestive System
Pancreatitis; diarrhea; vomiting; parotitis; nausea.
Hematologic and Lymphatic Systems
Thrombocytopenia; purpura; regional lymphadenopathy; leukocytosis.
Immune System
Anaphylaxis, anaphylactoid reactions, angioedema (including peripheral or facial edema) and bronchial spasm.
Musculoskeletal System
Arthritis; arthralgia; myalgia.
Nervous System
Encephalitis; encephalopathy; measles inclusion body encephalitis (MIBE) subacute sclerosing panencephalitis (SSPE); Guillain-Barré Syndrome (GBS); acute disseminated encephalomyelitis (ADEM); transverse myelitis; febrile convulsions; afebrile convulsions or seizures; ataxia; polyneuritis; polyneuropathy; ocular palsies; paresthesia.
Respiratory System
Pneumonia; pneumonitis; sore throat; cough; rhinitis.
Skin
Stevens-Johnson syndrome; acute hemorrhagic edema of infancy; Henoch-Schönlein purpura; erythema multiforme; urticaria; rash; measles-like rash; pruritus; injection site reactions (pain, erythema, swelling and vesiculation).
Special Senses — Ear
Nerve deafness; otitis media.
Special Senses — Eye
Retinitis; optic neuritis; papillitis; conjunctivitis.
Urogenital System
Epididymitis; orchitis.
7 DRUG INTERACTIONS
7.1 Corticosteroids and Immunosuppressive Drugs
M-M-R II vaccine should not be administered to individuals receiving immunosuppressive therapy, including high dose corticosteroids. Vaccination with M-M-R II vaccine can result in disseminated disease due to measles vaccine in individuals on immunosuppressive drugs [see Contraindications (4.2)].
7.2 Immune Globulins and Transfusions
Administration of immune globulins and other blood products concurrently with M-M-R II vaccine may interfere with the expected immune response {9-11} [see Warnings and Precautions (5.5)]. The ACIP has specific recommendations for intervals between administration of antibody containing products and live virus vaccines.
7.3 Tuberculin Skin Testing
It has been reported that live attenuated measles, mumps and rubella virus vaccines given individually may result in a temporary depression of tuberculin skin sensitivity. Therefore, if a tuberculin skin test with tuberculin purified protein derivative (PPD) is to be done, it should be administered before, simultaneously with, or at least 4 to 6 weeks after vaccination with M-M-R II vaccine.
7.4 Use with Other Live Viral Vaccines
M-M-R II vaccine can be administered concurrently with other live viral vaccines. If not given concurrently, M-M-R II vaccine should be given one month before or one month after administration of other live viral vaccines to avoid potential for immune interference.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
M-M-R II vaccine is contraindicated for use in pregnant women because infection during pregnancy with the wild-type viruses has been associated with maternal and fetal adverse outcomes.
Increased rates of spontaneous abortion, stillbirth, premature delivery and congenital defects have been observed following infection with wild-type measles during pregnancy. {12,13} Wild-type mumps infection during the first trimester of pregnancy may increase the rate of spontaneous abortion.
Infection with wild-type rubella during pregnancy can lead to miscarriage or stillbirth. If rubella infection occurs during the first trimester of pregnancy, it can result in severe congenital defects, Congenital Rubella Syndrome (CRS). Congenital Rubella Syndrome in the infant includes but is not limited to eye manifestations (cataracts, glaucoma, retinitis), congenital heart defects, hearing loss, microcephaly, and intellectual disabilities. M-M-R II vaccine contains live attenuated measles, mumps and rubella viruses. It is not known whether M-M-R II vaccine can cause fetal harm when administered to pregnant woman. There are no adequate and well-controlled studies of M-M-R II vaccine administration to pregnant women.
All pregnancies have a risk of birth defect, loss or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Available data suggest the rates of major birth defects and miscarriage in women who received M-M-R II vaccine within 30 days prior to pregnancy or during pregnancy are consistent with estimated background rates (see Data).
Human Data
A cumulative assessment of post-marketing reports for M-M-R II vaccine from licensure 01 April 1978 through 31 December 2018, identified 796 reports of inadvertent administration of M-M-R II vaccine occurring 30 days before or at any time during pregnancy with known pregnancy outcomes. Of the prospectively followed pregnancies for whom the timing of M-M-R II vaccination was known, 425 women received M-M-R II vaccine during the 30 days prior to conception through the second trimester. The outcomes for these 425 prospectively followed pregnancies included 16 infants with major birth defects, 4 cases of fetal death and 50 cases of miscarriage. No abnormalities compatible with congenital rubella syndrome have been identified in patients who received M-M-R II vaccine. Rubella vaccine virus can cross the placenta, leading to asymptomatic infection of the fetus. Mumps vaccine virus has also been shown to infect the placenta {14}, but there is no evidence that it causes congenital malformations or disease in the fetus or infant.
The CDC established the Vaccine in Pregnancy registry (1971-1989) of women who had received rubella vaccines within 3 months before or after conception. Data on 1221 inadvertently vaccinated pregnant women demonstrated no evidence of an increase in fetal abnormalities or cases of Congenital Rubella Syndrome (CRS) in the enrolled women {15}.
8.2 Lactation
Risk Summary
It is not known whether measles or mumps vaccine virus is secreted in human milk. Studies have shown that lactating postpartum women vaccinated with live attenuated rubella vaccine may secrete the virus in breast milk and transmit it to breast-fed infants. {16,17} In the breast-fed infants with serological evidence of rubella virus vaccine strain antibodies, none exhibited severe disease; however, one exhibited mild clinical illness typical of acquired rubella. {18,19}
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for M-M-R II, and any potential adverse effects on the breastfed child from M-M-R II or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
M-M-R II vaccine is not approved for individuals less than 12 months of age. Safety and effectiveness of measles vaccine in infants below the age of 6 months have not been established [see Clinical Studies (14)]. Safety and effectiveness of mumps and rubella vaccine in infants less than 12 months of age have not been established.
11 DESCRIPTION
M-M-R II vaccine is a sterile lyophilized preparation of (1) Measles Virus Vaccine Live, an attenuated line of measles virus, derived from Enders' attenuated Edmonston strain and propagated in chick embryo cell culture; (2) Mumps Virus Vaccine Live, the Jeryl Lynn™ (B level) strain of mumps virus propagated in chick embryo cell culture; and (3) Rubella Virus Vaccine Live, the Wistar RA 27/3 strain of live attenuated rubella virus propagated in WI-38 human diploid lung fibroblasts. {20,21} The cells, virus pools, recombinant human serum albumin and fetal bovine serum used in manufacturing are tested and determined to be free of adventitious agents.
After reconstitution, each 0.5 mL dose contains not less than 3.0 log10 TCID50 (tissue culture infectious doses) of measles virus; 4.1 log10 TCID50 of mumps virus; and 3.0 log10 TCID50 of rubella virus.
Each dose is calculated to contain sorbitol (14.5 mg), sucrose (1.9 mg), hydrolyzed gelatin (14.5 mg), recombinant human albumin (≤0.3 mg), fetal bovine serum (<1 ppm), approximately 25 mcg of neomycin and other buffer and media ingredients. The product contains no preservative.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
M-M-R II vaccination induces antibodies to measles, mumps, and rubella associated with protection which can be measured by neutralization assays, hemagglutination-inhibition (HI) assays, or enzyme linked immunosorbent assay (ELISA) tests. Results from efficacy studies or effectiveness studies that were previously conducted for the component vaccines of M-M-R II were used to define levels of serum antibodies that correlated with protection against measles, mumps, and rubella [see Clinical Studies (14)].
14 CLINICAL STUDIES
14.1 Clinical Efficacy
Efficacy of measles, mumps, and rubella vaccines was established in a series of double-blind controlled trials. {29-34} These studies also established that seroconversion in response to vaccination against measles, mumps and rubella paralleled protection. {35-38}
14.2 Immunogenicity
Clinical studies enrolling 284 triple seronegative children, 11 months to 7 years of age, demonstrated that M-M-R II vaccine is immunogenic. In these studies, a single injection of the vaccine induced measles HI antibodies in 95%, mumps neutralizing antibodies in 96%, and rubella HI antibodies in 99% of susceptible individuals.
A study of 6-month-old and 15-month-old infants born to mothers vaccinated with a measles vaccine in childhood, demonstrated that, following infant and toddler vaccination with Measles Virus Vaccine, Live (previously US-licensed, manufactured by Merck), 74% of the 6-month-old infants developed detectable neutralizing antibody titers while 100% of the 15-month-old infants vaccinated with Measles Virus Vaccine, Live or M-M-R II vaccine developed neutralizing antibodies {39}. When the 6-month-old infants of immunized mothers were revaccinated at 15 months with M-M-R II vaccine, they developed antibody titers similar to those of toddlers who were vaccinated previously at 15-months of age.
15 REFERENCES
- General Recommendations on Immunization, Recommendations of the Advisory Committee on Immunization Practices, MMWR 43(RR-1): 1-38, January 28, 1994.
- Measles, Mumps, and Rubella — Vaccine Use and Strategies for Elimination of Measles, Rubella, and Congenital Rubella Syndrome and Control of Mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP), MMWR 47(RR-8): May 22, 1998.
- Kelso, J.M.; Jones, R.T.; Yunginger, J.W.: Anaphylaxis to measles, mumps, and rubella vaccine mediated by IgE to gelatin, J. Allergy Clin. Immunol. 91: 867-872, 1993.
- Bitnum, A.; et al: Measles Inclusion Body Encephalitis Caused by the Vaccine Strain of Measles Virus. Clin. Infect. Dis. 29: 855-861, 1999.
- Angel, J.B.; et al: Vaccine Associated Measles Pneumonitis in an Adult with AIDS. Annals of Internal Medicine, 129: 104-106, 1998.
- Cecinati V, et al. Vaccine administration and the development of immune thrombocytopenic purpura in children. Human Vaccines & Immunotherapeutics 9:5, 2013.
- Mantadakis E, Farmaki E, Buchanan GR. Thrombocytopenic Purpura after Measles-Mumps-Rubella Vaccination: A Systematic Review of the Literature and Guidance for Management. J Ped 156(4): 2010.
- Andrews N, Stowe J, Miller E, Svanstrom H, Johansen K, Bonhoeffer J, et al. A collaborative approach to investigating the risk of thrombocytopenic purpura after measles-mumps-rubella vaccination in England and Denmark. Vaccine. 2012;30:3042-6.
- Rubella Prevention: Recommendation of the Immunization Practices Advisory Committee (ACIP), MMWR 39(RR-15): 1-18, November 23, 1990.
- Peter, G.; et al (eds): Report of the Committee on Infectious Diseases, Twenty-fourth Edition, American Academy of Pediatrics, 344-357, 1997.
- Measles Prevention: Recommendations of the Immunization Practices Advisory Committee (ACIP), MMWR 38(S-9): 5-22, December 29, 1989.
- Eberhart-Phillips, J.E.; et al: Measles in pregnancy: a descriptive study of 58 cases. Obstetrics and Gynecology, 82(5): 797-801, November 1993.
- Jespersen, C.S.; et al: Measles as a cause of fetal defects: A retrospective study of ten measles epidemics in Greenland. Acta Paediatr Scand. 66: 367-372, May 1977.
- Yamauchi T, Wilson C, Geme JW Jr. Transmission of live, attenuated mumps virus to the human placenta. N Engl J Med. 1974;290(13):710-712.
- Rubella Vaccination during Pregnancy —United States, 1971-1988. JAMA. 1989;261(23):3374–3383.
- Losonsky, G.A.; Fishaut, J.M.; Strussenber, J.; Ogra, P.L.: Effect of immunization against rubella on lactation products. II. Maternal-neonatal interactions, J. Infect. Dis. 145: 661-666, 1982.
- Losonsky, G.A.; Fishaut, J.M.; Strussenber, J.; Ogra, P.L.: Effect of immunization against rubella on lactation products. I. Development and characterization of specific immunologic reactivity in breast milk, J. Infect. Dis. 145: 654-660, 1982.
- Landes, R.D.; Bass, J.W.; Millunchick, E.W.; Oetgen, W.J.: Neonatal rubella following postpartum maternal immunization, J. Pediatr. 97: 465-467, 1980.
- Lerman, S.J.: Neonatal rubella following postpartum maternal immunization, J. Pediatr. 98: 668, 1981. (Letter)
- Plotkin, S.A.; Cornfeld, D.; Ingalls, T.H.: Studies of immunization with living rubella virus: Trials in children with a strain cultured from an aborted fetus, Am. J. Dis. Child. 110: 381-389, 1965.
- Plotkin, S.A.; Farquhar, J.; Katz, M.; Ingalls, T.H.: A new attenuated rubella virus grown in human fibroblasts: Evidence for reduced nasopharyngeal excretion, Am. J. Epidemiol. 86: 468-477, 1967.
- Weibel, R.E.; Carlson, A.J.; Villarejos, V.M.; Buynak, E.B.; McLean, A.A.; Hilleman, M.R.: Clinical and Laboratory Studies of Combined Live Measles, Mumps, and Rubella Vaccines Using the RA 27/3 Rubella Virus, Proc. Soc. Exp. Biol. Med. 165: 323-326, 1980.
- Watson, J.C.; Pearson, J.S.; Erdman, D.D.; et al: An Evaluation of Measles Revaccination Among School-Entry Age Children, 31st Interscience Conference on Antimicrobial Agents and Chemotherapy, Abstract #268, 143, 1991.
- Unpublished data from the files of Merck Research Laboratories.
- Davidkin, I.; Jokinen, S.; Broman, M. et al.: Persistence of Measles, Mumps, and Rubella Antibodies in an MMR-Vaccinated Cohort: A 20-Year Follow-up, JID 197:950–6, April 2008.
- LeBaron, W.; Beeler J.; Sullivan, B.; et al.: Persistence of Measles Antibodies After 2 Doses of Measles Vaccine in a Postelimination Environment, Arch Pediatr Adolesc Med. 161:294-301, March 2007.
- LeBaron, C.; Forghani, B.; Beck, C. et al.: Persistence of Mumps Antibodies after 2 Doses of Measles-Mumps-Rubella Vaccine, JID 199:552– 60 , February 2009.
- LeBaron, W.; Forghani, B.; Matter, L. et al.: Persistence of Rubella Antibodies after 2 Doses of Measles-Mumps-Rubella Vaccine, JID 200:888–99, September 2009.
- Hilleman, M.R.; Buynak, E.B.; Weibel, R.E.; et al: Development and Evaluation of the Moraten Measles Virus Vaccine, JAMA 206(3): 587-590, 1968.
- Weibel, R.E.; Stokes, J.; Buynak, E.B.; et al: Live, Attenuated Mumps Virus Vaccine 3. Clinical and Serologic Aspects in a Field Evaluation, N. Engl. J. Med. 276: 245-251, 1967.
- Hilleman, M.R.; Weibel, R.E.; Buynak, E.B.; et al: Live, Attenuated Mumps Virus Vaccine 4. Protective Efficacy as Measured in a Field Evaluation, N. Engl. J. Med. 276: 252-258, 1967.
- Cutts, F.T.; Henderson, R.H.; Clements, C.J.; et al: Principles of measles control, Bull WHO 69(1): 1-7, 1991.
- Weibel, R.E.; Buynak, E.B.; Stokes, J.; et al: Evaluation Of Live Attenuated Mumps Virus Vaccine, Strain Jeryl Lynn, First International Conference on Vaccines Against Viral and Rickettsial Diseases of Man, World Health Organization, No. 147, May 1967.
- Leibhaber, H.; Ingalls, T.H.; LeBouvier, G.L.; et al: Vaccination With RA 27/3 Rubella Vaccine, Am. J. Dis. Child. 123: 133-136, February 1972.
- Rosen, L.: Hemagglutination and Hemagglutination-Inhibition with Measles Virus, Virology 13: 139-141, January 1961.
- Brown, G.C.; et al: Fluorescent-Antibody Marker for Vaccine-Induced Rubella Antibodies, Infection and Immunity 2(4): 360-363, 1970.
- Buynak, E.B.; et al: Live Attenuated Mumps Virus Vaccine 1. Vaccine Development, Proceedings of the Society for Experimental Biology and Medicine, 123: 768-775, 1966.
- Hilleman M.R., Studies of Live Attenuated Measles Virus Vaccine in Man: II. Appraisal of Efficacy. Amer. J. of Public Health, 52(2):44-56, 1962.
- Johnson, C.E.; et al: Measles Vaccine Immunogenicity in 6- Versus 15-Month-Old Infants Born to Mothers in the Measles Vaccine Era, Pediatrics, 93(6): 939-943, 1994.
16 HOW SUPPLIED/STORAGE AND HANDLING
No. 4681 — M-M-R II vaccine is supplied as follows:
(1) a box of 10 single-dose vials of lyophilized vaccine (package A), NDC 0006-4681-00
(2) a box of 10 vials of diluent (package B)
Exposure to light may inactivate the vaccine viruses.
To maintain potency, M-M-R II must be stored between -58°F and +46°F (-50°C to +8°C). Use of dry ice may subject M-M-R II to temperatures colder than -58°F (-50°C).
Before reconstitution, refrigerate the lyophilized vaccine at 36°F to 46°F (2°C to 8°C).
Store accompanying diluent in the refrigerator (36°F to 46°F, 2°C to 8°C) or at room temperature (68°F to 77°F, 20°C to 25°C). Do not freeze the diluent.
Administer M-M-R II vaccine as soon as possible after reconstitution. If not administered immediately, reconstituted vaccine may be stored between 36°F to 46°F (2°C to 8°C), protected from light, for up to 8 hours. Discard reconstituted vaccine if it is not used within 8 hours.
For information regarding the product or questions regarding storage conditions, call 1-800-MERCK-90 (1-800-637-2590).
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Package Insert).
Discuss the following with the patient:
- Provide the required vaccine information to the patient, parent, or guardian.
- Inform the patient, parent, or guardian of the benefits and risks associated with vaccination.
- Question the patient, parent, or guardian about reactions to a previous dose of M-M-R II vaccine or other measles-, mumps-, or rubella-containing vaccines.
- Question females of reproductive potential regarding the possibility of pregnancy. Inform female patients to avoid pregnancy for 1 month following vaccination [see Contraindications (4.5) and Use in Specific Populations (8.1)].
- Inform the patient, parent, or guardian that vaccination with M-M-R II may not offer 100% protection from measles, mumps, and rubella infection.
- Instruct patients, parents, or guardians to report any adverse reactions to their health-care provider. The U.S. Department of Health and Human Services has established a Vaccine Adverse Event Reporting System (VAERS) to accept all reports of suspected adverse events after the administration of any vaccine, including but not limited to the reporting of events required by the National Childhood Vaccine Injury Act of 1986. For information or a copy of the vaccine reporting form, call the VAERS toll-free number at 1-800-822-7967, or report online at https://www.vaers.hhs.gov.
Distributed by: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
For patent information: www.merck.com/product/patent/home.html
Copyright © 1978-2020 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.
uspi-v205c-i-2012r011
|
Patient Information |
This is a summary of information about M-M-R® II. You should read it before you or your child receives the vaccine. If you have any questions about the vaccine after reading this leaflet, you should ask your health care provider. This is a summary only. It does not take the place of talking about M-M-R II with your doctor, nurse, or other health care provider. Only your health care provider can decide if M-M-R II is right for you or your child.
What is M-M-R II and how does it work?
M-M-R II is also known as Measles, Mumps, and Rubella Virus Vaccine Live. It is a live virus vaccine that is given as a shot. This vaccine is usually given to people one year old or older. It is meant to help prevent measles (rubeola), mumps, and rubella (German measles).
M-M-R II contains weakened forms of measles virus, mumps virus, and rubella virus.
M-M-R II works by helping the immune system protect you or your child from getting measles, mumps, or rubella.
M-M-R II may not protect everyone who gets the vaccine. M-M-R II does not treat measles, mumps, or rubella once you or your child has them.
What do I need to know about measles, mumps, and rubella?
Measles is also known as rubeola. It is a serious illness. Measles virus can be passed to others if you have it. Measles can give you a high fever, cough, and a rash. The illness can last for 1 to 2 weeks. In rare cases, it can also cause an infection of the brain. This could lead to seizures, hearing loss, intellectual disability, and even death.
Mumps can also be passed to others. This virus can cause fever and headache. It can also make the glands under your jaw swell and be painful. The illness often lasts for several days. Sometimes, mumps can make the testicles swell and be painful. In some cases, it can cause meningitis, which is a swelling of the coverings of the brain and spinal cord.
Rubella is also known as German measles. It is often a mild illness. Rubella virus can cause a mild fever, swollen glands in the neck, pain and swelling in the joints, and a rash that lasts for a short time. It can be very dangerous if a pregnant woman catches it. Women who catch German measles when they are pregnant can have babies who are stillborn. Also, the babies may be blind or deaf, or have heart disease or intellectual disability.
Who should not get M-M-R II?
Do not get M-M-R II if you or your child:
- are allergic to any of its ingredients. (This includes gelatin. See the ingredient list at the end of this leaflet.)
- have a weakened immune system (which includes taking high doses of steroids by mouth or in a shot).
- have a fever.
- have active tuberculosis that is not treated.
- are pregnant or plan to get pregnant within the next month.
What should you tell your health care provider before getting M-M-R II?
Tell your health care provider if you or your child:
- have or have had any medical problems.
- have a history of seizures or someone in your family has a history of seizures.
- have received blood or plasma transfusions or human serum globulin.
- take any medicines. (This includes non-prescription medicines and dietary supplements.)
- have any allergies.
- had an allergic reaction to any other vaccine.
- have or have had a low blood platelet count.
- are allergic to eggs.
How is M-M-R II given?
M-M-R II is given as a shot to people one year old or older. The dose of the vaccine is the same for everyone. If your child gets the shot when he or she is one year old or older, a second dose is recommended. Often, the second dose is given right before the child goes to elementary school (4 to 6 years of age), but may be given earlier as long as the second dose is at least one month after the first dose.
If your child is less than one year old when he or she first gets the shot, a second dose should be given when they are 12 to 15 months old. Then, a third shot should be given between 4 and 6 years of age. Your doctor will decide the best time and number of shots by using official recommendations.
If a dose is missed, your health care provider will let you know when you should have it.
What are the possible side effects of M-M-R II?
The most common side effect of vaccination with M-M-R II is pain at the site of the shot for a short time.
Other side effects may include:
- Fever
- Rash
Less common side effects may also include:
- Swelling of the testicles
- Joint pain and/or swelling
Some side effects are rare but may be serious. You should call your health care provider if you notice any of the following problems:
- Difficulty breathing, wheezing, hives, or a skin rash may be signs of an allergic reaction
- Bleeding or bruising under the skin
- Seizures, a severe headache, a change in behavior or consciousness, or difficulty walking
Other side effects may also occur. Your doctor has a more complete list of side effects for M-M-R II.
Contact your doctor or health care provider if you or your child have any new or unusual symptoms after receiving M-M-R II.
Report the following to your doctor or your child’s doctor:
- exposure to M-M-R II during pregnancy
- exposure to M-M-R II during the month before getting pregnant
You may also report any adverse reactions to your doctor or your child's health care provider or submit a report directly to the Vaccine Adverse Event Reporting System (VAERS). The VAERS toll-free number is 1-800-822-7967 or you may report online to www.vaers.hhs.gov.
What are the ingredients of M-M-R II?
Active Ingredients: weakened forms of the measles, mumps, and rubella viruses.
Inactive Ingredients: sorbitol, sucrose, hydrolyzed gelatin, recombinant human albumin, fetal bovine serum, other buffer and media ingredients, neomycin.
What else should I know about M-M-R II?
This leaflet summarizes important information about M-M-R II.
If you would like more information, talk to your health care provider or call 1-800-622-4477.
Dist. by: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
For patent information: www.merck.com/product/patent/home.html
Copyright © 1978-2020 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.
usppi-v205c-i-2006r205
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 06/2020
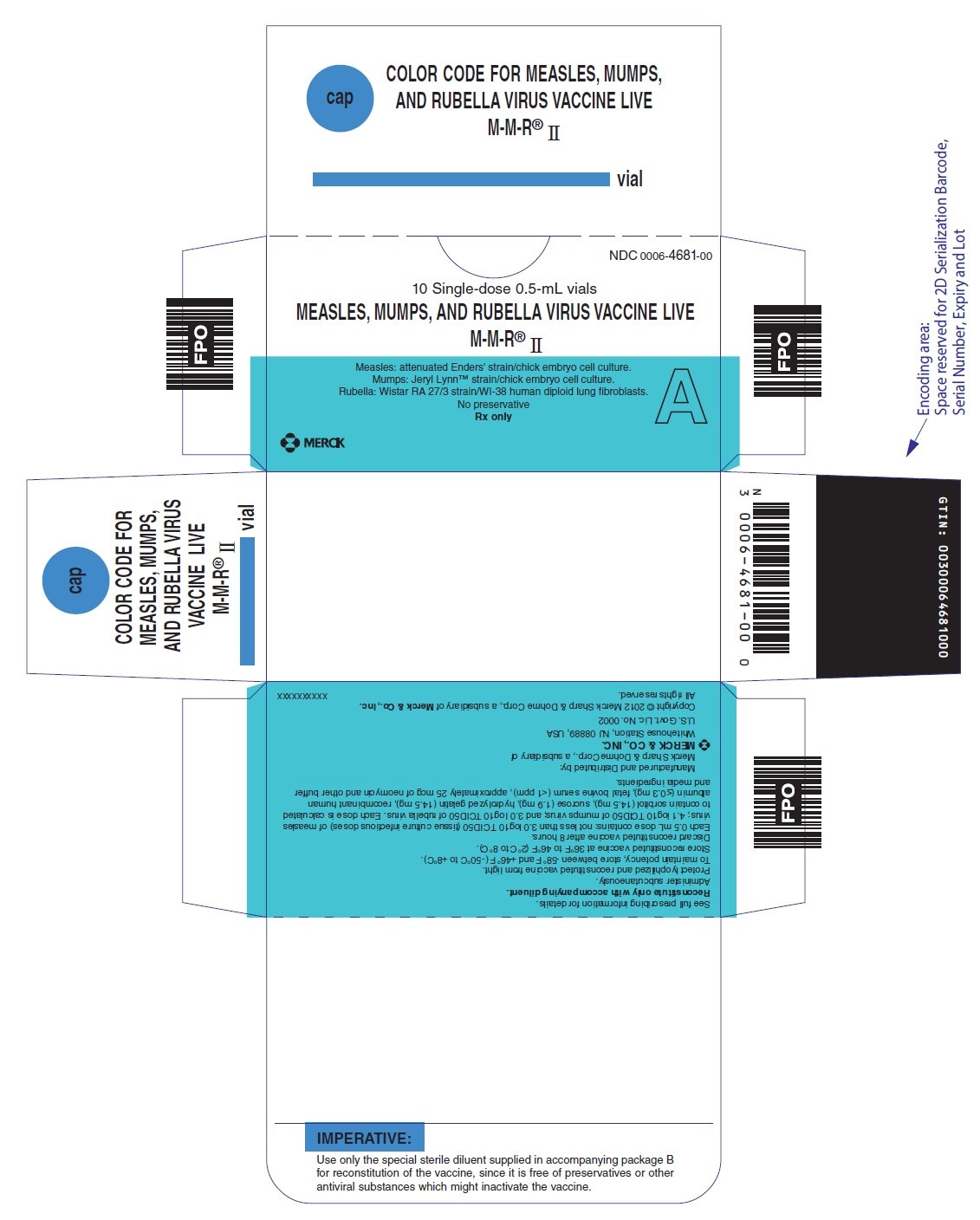
PRINCIPAL DISPLAY PANEL - 0.5 mL Vial Carton
NDC 0006-4681-00
10 Single-dose 0.5-mL vials
MEASLES, MUMPS, AND RUBELLA VIRUS VACCINE LIVE
M-M-R® II
Measles: attenuated Enders' strain/chick embryo cell culture.
Mumps: Jeryl Lynn™ strain/chick embryo cell culture.
Rubella: Wistar RA 27/3 strain/WI-38 human diploid lung fibroblasts.
No preservative
Rx only
A
| M-M-R II
measles, mumps, and rubella virus vaccine live injection, powder, lyophilized, for suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Merck Sharp & Dohme Corp. (001317601) |