TOTECT- dexrazoxane
TopoTarget USA
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Totect® safely and effectively. See full prescribing information for Totect®.
Totect® Kit (dexrazoxane) for injection, for intravenous infusion only Initial U.S. Approval: 2007 INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONSNone (4) WARNINGS AND PRECAUTIONSADVERSE REACTIONSThe most common adverse reactions (≥16%) are nausea, pyrexia, injection site pain and vomiting. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Topotarget Medical Information at 1-866-914-2922 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION. Revised: 8/2013 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Totect® is indicated for the treatment of extravasation resulting from intravenous anthracycline chemotherapy.
2 DOSAGE AND ADMINISTRATION
Vial contents must be mixed and diluted before use.
2.1 Recommended Dose
Totect® should be given once daily for 3 consecutive days. The first infusion should be initiated as soon as possible and within the first six hours after extravasation.
The individual dosage is based on calculation of the Body Surface Area (BSA) up to a maximum dose of 2000 mg (each on Day 1 and 2) and 1000 mg (Day 3), corresponding to a BSA of 2 m2.
| The recommended dose is: | Maximum daily dose: |
| Day one: 1000 mg/m2 | 2000 mg |
| Day two: 1000 mg/m2 | 2000 mg |
| Day three: 500 mg/m2 | 1000 mg |
2.2 Dose Modifications
The Totect® dose should be reduced by 50% in patients with creatinine clearance values < 40 mL/min.
2.3 Directions for Mixing and Final Dilution
Read this entire section carefully before mixing and diluting.
Aseptic technique should be used during preparation.
Caution must be exercised when handling Totect® and preparing the mixed solution. [see How Supplied/Storage and Handling (16)]
Totect® should not be mixed or administered with any other drug during the infusion.
Preparation of Totect®
Step 1. Each vial of Totect® (dexrazoxane for injection) (500 mg) must first be mixed with 50 mL of the enclosed diluent. The resultant solution contains 10 mg/mL. This resultant solution should be used immediately (within 2 hours) after preparation. It contains no antibacterial preservative.
Step 2. Withdraw the recommended dose from the solution containing 10 mg/mL as prepared in Step 1 and further dilute into an infusion bag containing 1000 mL 0.9% Sodium Chloride. In order to obtain the required dose more than one vial may be needed. Totect® must not be mixed with any other drugs.
The infusion bag should be used immediately after preparation. The product is stable for 4 hours from the time of preparation when stored below 25ºC (77ºF).
The solution of Totect® is slightly yellow.
Parenteral drug products should be inspected visually for particulate matter prior to administration, whenever solution and container permit. Solutions containing a precipitate should be discarded. Vials are for single use only. Unused solution should be discarded.
2.4 Administration
Totect® should not be mixed or administered with any other drug during the infusion. Administer as an intravenous infusion over 1 to 2 hours at room temperature and normal light conditions in a large caliber vein in an extremity/area other than the one affected by the extravasation. Cooling procedures such as ice packs, if used, should be removed from the extravasation area at least 15 minutes before Totect® administration in order to allow sufficient blood flow to the area of extravasation. Treatment on Day 2 and Day 3 should start at the same hour (+/- 3 hours) as on the first day.
3 DOSAGE FORMS AND STRENGTHS
Totect® is packaged as an urgent treatment kit for single patient use. Each kit contains 10 vials of Totect® (dexrazoxane for injection) 500 mg and 10 vials of 50 mL diluent, which provides a complete three day treatment.
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
Treatment with Totect® is associated with leukopenia, neutropenia, and thrombocytopenia. Hematological monitoring should be performed.
5.2 Use in Pregnancy
Pregnancy Category D
Totect® can cause fetal harm when administered to a pregnant woman. There is no adequate information about the use of Totect® in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. [see Use in Special Populations (8.1)].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed cannot be directly compared to rates in other trials and may not reflect the rates observed in clinical practice.
In the clinical studies, Totect® was administered to patients also receiving chemotherapeutic agents for cancer, and the adverse reaction profile reflects the combination of Totect®, underlying disease, and already administered chemotherapy. The adverse reaction data reflect exposure to Totect® in 80 patients who received the first dose, 72 patients who received two doses, and 69 patients who received all three doses. Table 1 summarizes adverse reactions occurring with ≥ 5% frequency.
| MedDRA System Organ Class (SOC) and Preferred term | Study 1 and 2 Combined (All causalities) N=80 (%) |
|---|---|
| Total number of patients with at least one event | 68 (85) |
| General disorders and administration site conditions | 46 (58) |
| Pyrexia | 17 (21) |
| Injection site pain/injection site discomfort | 13 (16) |
| Fatigue | 10 (13) |
| Edema peripheral | 8 (10) |
| Injection site phlebitis | 5 (6) |
| Gastrointestinal disorders | 44 (55) |
| Nausea | 34 (43) |
| Vomiting | 15 (19) |
| Diarrhea | 9 (11) |
| Abdominal pain | 5 (6) |
| Constipation | 5 (6) |
| Infections and infestations | 24 (30) |
| Postoperative infection | 13 (16) |
| Nervous system disorders | 19 (24) |
| Dizziness | 9 (11) |
| Headache | 5 (6) |
| Skin and subcutaneous disorders | 14 (18) |
| Alopecia | 11 (14) |
| Respiratory, thoracic and mediastinal disorders | 13 (16) |
| Dyspnea | 6 (8) |
| Pneumonia Cough | 5 (6) 4 (5) |
| Vascular disorders | 12 (15) |
| Blood and lymphatic system disorders | 11 (14) |
| Anemia | 5 (6) |
| Psychiatric disorders | 11 (14) |
| Depression Insomnia | 6 (8) 4 (5) |
| Musculoskeletal and connective tissue disorders | 10 (13) |
| Metabolism and nutrition disorders Anorexia | 8 (10) 4 (5) |
| Cardiac disorders | 4 (5) |
Neutropenia and febrile neutropenia each occurred in 2.5% of patients.
Table 2 summarizes laboratory adverse events from studies 1 and 2.
| CTCAE version 3 Term | CTC grade 3 | CTC grade 4 | CTC grade 2 to 4 |
| N (%) | N (%) | N (%) | |
| Hematologic: | |||
| Decreased hemoglobin | 2 (3) | 0 | 34 (43) |
| Decreased WBC | 20 (25) | 16 (20) | 58 (73) |
| Decreased neutrophils | 17 (22) | 19 (24) | 48 (61) |
| Decreased platelets | 17 (21) | 0 | 21 (26) |
| Hepatic: | |||
| Increased bilirubin | 1 (2) | 0 | 6 (11) |
| Increased AST | 1 (1) | 1 (1) | 21 (28) |
| Increased ALT | 1 (1) | 4 (5) | 17 (22) |
| Increased alkaline phosphatase | 0 | 0 | 3 (4) |
| Increased LDH | 0 | 0 | 1 (5) |
| Metabolic: | |||
| Increased creatinine | 1 (2) | 1 (2) | 8 (14) |
| Decreased sodium | 4 (5) | 1 (1) | 5 (6) |
| Increased calcium total | 1 (2) | 1 (2) | 4 (7) |
7 DRUG INTERACTIONS
No drug interactions have been identified [see Clinical Pharmacology (12.3)].
-
Dimethylsulfoxide: Based on anecdotal reports concurrent use of topical dimethyl sulfoxide (DMSO) at the site of tissue injury may reduce the benefit of Totect®. Additionally, nonclinical studies using a mouse model that simulates extravasation of anthracyclines has shown that concomitant treatment with topical DMSO decreases the efficacy of systemic dexrazoxane.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category D [see Warnings and Precautions (5.2)].
Dexrazoxane was toxic to pregnant rats at doses of 2 mg/kg (1/80 the human dose on an mg/m2 basis) and embryotoxic and teratogenic at 8 mg/kg (about 1/20 the human dose on an mg/m2 basis) when given daily during the period of organogenesis. Teratogenic effects in the rat included imperforate anus, microphthalmia, and anophthalmia. In offspring allowed to develop to maturity, fertility was impaired in the male and female rats treated in utero during organogenesis at 8 mg/kg. In rabbits, doses of 5 mg/kg (about 1/16 the human dose on an mg/m2 basis) daily during the period of organogenesis caused maternal toxicity and doses of 20 mg/kg (1/4 the human dose on an mg/m2 basis) were embryotoxic and teratogenic. Teratogenic effects in the rabbit included several skeletal malformations such as short tail, rib and thoracic malformations, and soft tissue variations including subcutaneous, eye and cardiac hemorrhagic areas, as well as agenesis of the gallbladder and of the intermediate lobe of the lung.
8.3 Nursing Mothers
It is not known whether dexrazoxane or its metabolites are excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from dexrazoxane, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and effectiveness of Totect® in pediatric patients have not been established.
8.5 Geriatric Use
In total, 21% of the patients treated with Totect® were age 65 years or older and 9% were 75 and older. No differences in safety or efficacy were observed between older and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Dosage and Administration (2.2)].
8.6 Renal Impairment
Greater exposure to dexrazoxane may occur in patients with compromised renal function. The Totect® dose should be reduced by 50% in patients with creatinine clearance values < 40 mL/min. [see Dosage and Administration (2.2)]
11 DESCRIPTION
Totect® (dexrazoxane for injection) is a sterile, pyrogen-free lyophilizate intended for intravenous (IV) administration. Totect® is packaged as a kit for single patient use. Each kit contains 10 vials of Totect® (dexrazoxane for injection) 500 mg and 10 vials of 50 mL diluent, which provides a complete three day treatment.
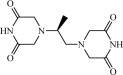
Chemically, dexrazoxane is 2,6-piperazinedione,4,4'-(1-methyl-1,2-ethanediyl)bis-,(S)- or (S)-(+)-1,2-bis(3,5-dioxopiperazin-1-yl)propane. The following diagram shows the chemical structure:
The molecular formula is C11H16N4O4; the molecular weight is 268.3. Dexrazoxane is a white to off-white powder, with a melting point of 194 ± 3 °C. It is soluble in dioxane and 0.1 N HCl, sparingly soluble in water, tetrahydrofuran, citrate buffer at pH 4.0, phosphate buffer at pH 7.0, and borate-potassium chloride sodium hydroxide buffer at pH 9.0. The acid dissociation constants, pKa, are 2.5 (for the tertiary piperazine nitrogen) and 9.7 (for the nitrogen imide). Log P is -2.135.
The finished product is supplied in a sterile form for intravenous infusion only following mixing and diluting.
Each kit contains twenty 50 mL Type I glass vials. Ten vials each contains dexrazoxane hydrochloride equivalent to 500 mg dexrazoxane and 10 vials each contains diluent (0.167M Sodium Lactate Injection, USP). Each vial of dexrazoxane for injection is closed with an aluminum flip-off cap covered with a dark red overcap. Each vial of diluent is closed with an aluminum flip-off cap covered with a white overcap.
When reconstituted as directed, the admixture contains dexrazoxane and the following excipients: hydrochloric acid, sodium lactate, water for injection, sodium hydroxide and lactic acid [see Dosage and Administration (2.4)]. The admixture should be further diluted in 0.9 % NaCl prior to administration to patients.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism by which Totect® diminishes tissue damage resulting from the extravasation of anthracycline drugs is unknown. Some evidence suggests that dexrazoxane inhibits topoisomerase II reversibly.
12.3 Pharmacokinetics
The pharmacokinetics of dexrazoxane have been studied in advanced cancer patients with normal renal and hepatic function. Generally, the pharmacokinetics of dexrazoxane can be adequately described by a two-compartment open model with first-order elimination. Dexrazoxane has been administered as a 15 minute infusion over a dose-range of 60 to 900 mg/m2 with 60 mg/m2 of doxorubicin, and at a fixed dose of 500 mg/m2 with 50 mg/m2 doxorubicin. The disposition kinetics of dexrazoxane are dose-independent, as shown by linear relationship between the area under plasma concentration-time curves and administered doses ranging from 60 to 900 mg/m2. The mean peak plasma concentration of dexrazoxane was 36.5 μg/mL at the end of the 15 minute infusion of a 500 mg/m2 dose of dexrazoxane administered 15 to 30 minutes prior to the 50 mg/m2 doxorubicin dose. The important pharmacokinetic parameters of dexrazoxane are summarized in the following table.
|
a Coefficient of variation |
||||||
|
b Steady-state volume of distribution |
||||||
| Dose Doxorubicin (mg/m2) | Dose Dexrazoxane (mg/m2) | Number of Subjects | Elimination Half-Life (h) | Plasma Clearance (L/h/m2) | Renal Clearance (L/h/m2) | bVolume of Distribution (L/m2) |
| 50 | 500 | 10 | 2.5 (16) | 7.88 (18) | 3.35 (36) | 22.4 (22) |
| 60 | 600 | 5 | 2.1 (29) | 6.25 (31) | — | 22.0 (55) |
Following a rapid distributive phase (~0.2 to 0.3 hours), dexrazoxane reaches post-distributive equilibrium within 2 to 4 hours. The estimated steady-state volume of distribution of dexrazoxane suggests its distribution primarily in the total body water (25 L/m2).
In a study of the pharmacokinetics of dexrazoxane following the recommended dosing for patients with anthracycline extravasation, the mean systemic clearance and steady-state volume of distribution of dexrazoxane in six female patients undergoing treatment for anthracycline extravasations at a dose of 1000 mg/m2 Totect® on Days 1 and 2 and 500 mg/m2 on Day 3 were similar to that observed when administered with doxorubicin. The systemic clearances (mean ± SD) were similar among Day 1 (5.9 ± 2.0 L/h/m2), Day 2 (6.4 ± 2.1 L/h/m2), and Day 3 (7.9 ± 3.0 L/h/m2). The terminal elimination half life did not change over 3 days (2.1-2.2 h). The volume of distribution was 17.9 ~ 22.6 L/m2.
Qualitative metabolism studies with dexrazoxane have confirmed the presence of unchanged drug, a diacid-diamide cleavage product, and two monoacid-monoamide ring products in the urine of animals and man. The metabolite levels were not measured in the pharmacokinetic studies.
Urinary excretion plays an important role in the elimination of dexrazoxane. Forty-two percent of the 500 mg/m2 dose of dexrazoxane was excreted in the urine.
Effects of Gender
There are no clinically relevant differences in the pharmacokinetics of dexrazoxane between males and females.
Renal insufficiency
The pharmacokinetics of dexrazoxane were assessed following a single 15 minute IV infusion of 150 mg/m2 of dexrazoxane in male and female subjects with varying degrees of renal dysfunction as determined by creatinine clearance (CLCR) based on a 24-hour urinary creatinine collection. Dexrazoxane clearance was reduced in subjects with renal dysfunction. Compared with controls, the mean AUC0-inf value was twofold greater in subjects with moderate (CLCR 30-50 mL/min) to severe (CLCR < 30 mL/min) renal dysfunction. Modeling demonstrated that equivalent exposure (AUC0-inf) could be achieved if dosing were reduced by 50% in subjects with creatinine clearance values < 40 mL/min compared with control subjects (CLCR > 80 mL/min) [see Dosage and Administration (2.2)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of dexrazoxane has not been investigated. Nevertheless, a study by the National Cancer Institute has reported that long term dosing with razoxane (the racemic mixture of dexrazoxane, ICRF-187, and its enantiomer ICRF-186) is associated with the development of malignancies in rats and possibly in mice. Dexrazoxane was not mutagenic to bacteria in vitro (Ames assay), but caused significant chromosomal aberrations in mammalian cells in vitro. It also increased the formation of micronucleated polychromatic erythrocytes in mice. Thus, dexrazoxane is mutagenic and clastogenic.
The possible adverse effects of Totect® on the fertility of humans and experimental animals, male or female, have not been adequately studied. Testicular atrophy was seen with dexrazoxane administration at doses as low as 30 mg/kg weekly for 6 weeks in rats (about 1/5 the human dose on a mg/m2 basis) and as low as 20 mg/kg weekly for 13 weeks in dogs (about half the human dose on a mg/m2 basis).
14 CLINICAL STUDIES
Totect® was studied in two open-label, single arm, multi-center studies testing whether Totect® administration could reduce tissue injury following anthracycline extravasation and thereby reduce or avoid surgical intervention.
In the studies, eligible patients were receiving single-agent anthracycline intravenously (usually as part of combination chemotherapy) and developed extravasation symptoms of pain, burning, swelling, and/or redness near the infusion site. Skin biopsy samples from the suspected skin area were examined for the presence of anthracycline as determined by the presence of tissue fluorescence; however, therapy was not delayed for this test result.
In both studies, treatment with Totect® was to begin as soon as possible and no later than 6 hours after extravasation with retreatment 24 and 48 hours later (a total of 3 doses). Totect® was administered as 1-2 hour IV infusions through a different venous access location. The first and second doses were 1000 mg/m2 and the third dose was 500 mg/m2. No dose modifications were planned except for patients whose body surface area exceeded 2.0 m2, in which case the total daily dose limit on the first and second day was 2000 mg/day and 1000 mg on the third day.
In total, 80 patients were enrolled and 57 were evaluable. Demographics in the two studies were similar. The median age was 57 years, and sixty-five percent of patients were women. The anthracyclines most commonly associated with extravasation were epirubicin (56%) and doxorubicin (41%). Peripheral IV sites of extravasation included the forearm in 63%, the hand in 21%, and the antecubital area in 11%; four patients (5%) received the anthracycline via a central venous access device (CVAD). Most patients presented with swelling (83%), redness (78%), and pain (43%). The median baseline lesion area was 25 cm2 (range 1-253 cm2).
Evaluable patients had to be receiving IV anthracycline (single agent or in combination) at the time of extravasation, to have skin biopsies showing fluorescence, and to receive the first Totect® dose within 6 hours of the extravasation.
In study 1, none of the 19 evaluable patients required surgical intervention and none had serious late sequelae. In study 2, one of the 38 evaluable patients required surgery. One additional non-evaluable patient required surgery for tissue necrosis. Thirteen patients had late sequelae at the event site such as site pain, fibrosis, atrophy, and local sensory disturbance; all were judged as mild except in the one patient who required surgery. None of the 4 patients with CVADs required surgical intervention.
15 REFERENCES
- NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. 2004. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004-165.
- OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
- American Society of Health-System Pharmacists. ASHP guidelines on handling hazardous drugs. Am J Health-Syst Pharm. 2006;63:1172-1193.
- Polovich, M., White, J.M., & Kelleher, L.O. (eds.) 2005. Chemotherapy and biotherapy guidelines and recommendations for practice (2nd. ed.) Pittsburgh, PA: Oncology Nursing Society
16 HOW SUPPLIED/STORAGE AND HANDLING
Totect® is available as an urgent treatment kit for single patient use. Each kit contains 10 vials of Totect® (dexrazoxane for injection) 500 mg and 10 vials of 50 mL diluent, which provides a complete three day treatment.
NDC 38423-110-01
Store at 25ºC (77ºF); excursions permitted between 15-30ºC (59-86ºF) [see USP Controlled Room Temperature]. Protect from light. Keep vials in carton until ready for use.
Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published.1-4 Direct contact of Totect® with the skin or mucous membrances prior to and following reconstitution should be avoided. If contact occurs, wash immediately and thoroughly with water.
Rx Only
17 PATIENT COUNSELING INFORMATION
See FDA-approved Patient Labeling (17.3)
17.1 Myelosuppression
Treatment with Totect® is associated with leukopenia, neutropenia, and thrombocytopenia. Perform hematological monitoring. [see Warnings and Precautions (5.1)]
17.2 Pregnancy
Women who have potential to become pregnant should be advised that Totect® might cause fetal harm. [see Warnings and Precautions (5.3)]
17.3 FDA-Approved Patient Labeling
PATIENT INFORMATION
TOTECT®
(dex-ra-ZOX-ane)
(dexrazoxane)
Injection
Read the Patient Information that comes with Totect® before you receive treatment. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is Totect®?
Totect® is a prescription medicine used to treat people when anthracycline chemotherapy leaks from your vein into the tissue around the intravenous (IV) site.
Totect® has not been studied in children.
What should I tell my healthcare provider before receiving Totect®?
Before receiving Totect®:
- -
- tell your healthcare provider about all your medical conditions including if you have any kidney problems.
- -
- tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, herbal or dietary supplements. Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
- -
- tell your healthcare provider if you use a topical dimethylsulfoxide (DMSO). Topical DMSO should not be used in combination with Totect® since it may lessen the effect of Totect®.
- -
- tell your healthcare provider if you are pregnant, could be pregnant, or are planning to become pregnant, or are breast-feeding.
It is not known if Totect® passes into your breast milk. You and your doctor should decide if you will receive Totect® or breast feed. You should not do both. Talk to your doctor about the best way to feed your baby if you take Totect®. Do not breast feed while taking Totect®.
How will I receive Totect®?
- -
- Totect® is given to you in your healthcare provider's office, clinic or hospital.
- -
- Totect® is infused into a vein for 1 to 2 hours each day for three days.
What are the possible side effects of Totect®?
Totect® can cause serious side effects including:
- a decrease in white blood cell counts (leukopenia and neutropenia)
- a decrease in the blood cells which help your blood to clot (thrombocytopenia)
Blood tests may be needed to monitor for these side effects.
Common side effects:
- nausea
- fever
- pain at the intravenous site
- vomiting
Tell your healthcare provider if you have any side effect that bothers you or does not go away.
These are not all the possible side effects of Totect®. For more information, ask your healthcare provider or pharmacist.
Talk to your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088 (1-800-332-1088) or at www.fda.gov/medwatch.
General Information about Totect®
Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet.
This Patient Information summarizes the most important information about Totect®. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Totect® that is written for health professionals.
For more information, go to www.totect.com or call 1-866-914-2922.
What are the ingredients in Totect®?
Active ingredient: dexrazoxane
Inactive ingredients: hydrochloric acid, sodium lactate, water for injection, sodium hydroxide and lactic acid
Marketed by:
Topotarget USA Inc.
100 Enterprise Drive
Rockaway, New Jersey 07866
USA
Distributed and Packaged by:
Integrated Commercialization Solutions
Brooks, KY 40109
USA
See www.totect.com webpage for the distributors
Manufactured by:
Ben Venue Laboratories, Inc.
Bedford, Ohio 44146
USA
Hameln Pharmaceuticals GmbH
31789 Hameln
Germany
Manufactured for:
Topotarget A/S
Symbion Science Park
Fruebjergvej 3
DK-2100 Copenhagen
Denmark
Totect® is a registered trademark of Topotarget A/S, Copenhagen, Denmark.
US Patent No 6,727,253 B2
May 2011
TOT-PIL/v05
BOX LABEL – PRINCIPAL DISPLAY PANEL
NDC 38423-110-01
Topotarget logo
TOTECT®
(dexrazoxane) for injection
500 mg
Sterile product
For Intravenous Use Following Reconstitution and Dilution Only
Vials are for single use only. Unused solution should be discarded.
After mixing with 50 mL Diluent for Totect® 1 mL contains 10 mg dexrazoxane.
See package insert for information on dosage and preparation for use.
KIT FOR ANTHRACYCLINE EXTRAVASATION
Each Totect® vial contains dexrazoxane hydrochloride equivalent to 500 mg dexrazoxane. Each vial of Diluent for Totect® contains Sodium lactate 50% 1.87 g, Lactic Acid q.s., Sodium hydroxide q.s., Water for injection q.s. ad 50 mL.
| TOTECT
dexrazoxane kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - TopoTarget USA (790709161) |
| Registrant - Biocodex USA (792454464) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ben Venue Laboratories, Inc. | 004327953 | MANUFACTURE(38423-110), ANALYSIS(38423-110) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hameln Pharmaceuticals GmbH | 315869123 | MANUFACTURE(38423-110), ANALYSIS(38423-110) | |