IQUIX- levofloxacin solution
Vistakon Pharmaceuticals LLC
----------
IQUIX®
(levofloxacin ophthalmic solution) 1.5%
DESCRIPTION
IQUIX® (levofloxacin ophthalmic solution) 1.5% is a sterile topical ophthalmic solution. Levofloxacin is a fluoroquinolone antibacterial active against a broad spectrum of Gram-positive and Gram-negative ocular pathogens. Levofloxacin is a fluorinated 4-quinolone containing a six-member (pyridobenzoxazine) ring from positions 1 to 8 of the basic ring structure. Levofloxacin is the pure(-)-(S)-enantiomer of the racemic drug substance, ofloxacin. It is more soluble in water at neutral pH than ofloxacin.
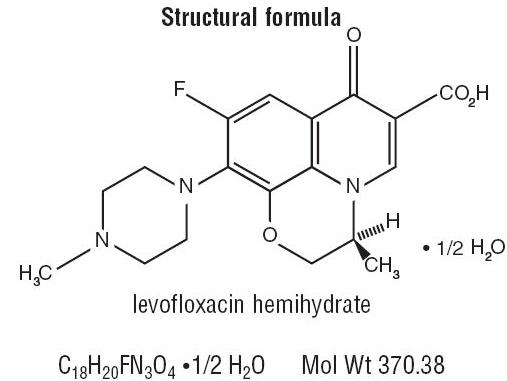
Chemical Name: (-)(S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4 benzoxazine-6-carboxylic acid hemihydrate.
Levofloxacin (hemihydrate) is a yellowish-white crystalline powder.
Each mL of IQUIX® contains 15.36 mg of levofloxacin hemihydrate equivalent to 15 mg levofloxacin.
CLINICAL PHARMACOLOGY
Pharmacokinetics
Levofloxacin concentration in plasma was measured in 14 healthy adult volunteers during a 16-day course of treatment with IQUIX® solution. The dosing schedule was 1-2 drops per eye once in the morning on Days 1 and 16; 1-2 drops per eye every two hours Days 2 through 8; and 1-2 drops per eye every four hours Days 9 through 15. The mean levofloxacin concentration in plasma 1 hour postdose ranged from 3.13 ng/mL on Day 1 to 10.4 ng/mL on Day 16. Maximum mean levofloxacin concentrations increased from 3.22 ng/mL on Day 1 to 10.9 ng/mL on Day 16, which is more than 400 times lower than those reported after standard oral doses of levofloxacin.
Levofloxacin concentration in tears was measured in 100 healthy adult volunteers at various time points following instillation of 2 drops of IQUIX® solution. Mean tear concentration measured 15 minutes after instillation was 757 µg/mL.
Microbiology
Levofloxacin is the L-isomer of the racemate, ofloxacin, a quinolone antimicrobial agent. The antibacterial activity of ofloxacin resides primarily in the L-isomer. The mechanism of action of levofloxacin and other fluoroquinolone antimicrobials involves the inhibition of bacterial topoisomerase IV and DNA gyrase (both of which are type II topoisomerases), enzymes required for DNA replication, transcription, repair, and recombination.
Levofloxacin has in vitro activity against a wide range of Gram-negative and Gram-positive microorganisms and is often bactericidal at concentrations equal to or slightly greater than inhibitory concentrations.
Fluoroquinolones, including levofloxacin, differ in chemical structure and mode of action from β-Iactam antibiotics and aminoglycosides, and therefore may be active against bacteria resistant to β-Iactarn antibiotics and aminoglycosides. Additionally, β-Iactam antibiotics and aminoglycosides may be active against bacteria resistant to levofloxacin. Resistance to levofloxacin due to spontaneous mutation in vitro is a rare occurrence (range: 10-9 to 10-10).
Levofloxacin has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section:
| AEROBIC GRAM-POSITIVE MICROORGANISMS: | AEROBIC GRAM-NEGATIVE MICROORGANISMS: |
|---|---|
|
|
| Corynebacterium species * | Pseudomonas aeruginosa |
| Staphylococcus aureus | Serratia marcescens* |
| Staphylococcus epidermidis | |
| Streptococcus pneumoniae | |
| Viridans group streptococci * | |
The following in vitro data are also available, but their clinical significance in ophthalmic infections is unknown. The safety and effectiveness of levofloxacin in treating ophthalmological infections due to these microorganisms have not been established in adequate and well-controlled trials.
These organisms are considered susceptible when evaluated using systemic breakpoints. However, a correlation between the in vitro systemic breakpoint and ophthalmological efficacy has not been established. The list of organisms is provided as guidance only in assessing the potential treatment of corneal ulcer. Levofloxacin exhibits in vitro minimal inhibitory concentrations (MICs) of 2 µg/mL or less (systemic susceptible breakpoint) against most (≥90%) strains of the following ocular pathogens:
| AEROBIC GRAM-POSITIVE MICROORGANISMS: |
|---|
| Enterococcus faecalis (many strains are only moderately susceptible) |
| Staphylococcus saprophyticus |
| Streptococcus agalactiae |
| Streptococcus pyogenes |
| Streptococcus (Group C/F) |
| Streptococcus (Group G) |
| AEROBIC GRAM-NEGATIVE MICROORGANISMS: | |
|---|---|
| Acinetobacter baumannii | Legionella pneumophila |
| Acinetobacter Iwoffii | Moraxella catarrhalis |
| Citrobacter koseri | Morganella morganii |
| Citrobacter freundii | Neisseria gonorrhoeae |
| Enterobacter aerogenes | Pantoea agglomerans |
| Enterobacter cloacae | Proteus mirabilis |
| Escherichia coli | Proteus vulgaris |
| Haemophilus influenzae | Providencia rettgeri |
| Haemophilus parainfluenzae | Providencia stuartii |
| Klebsiella oxytoca | Pseudomonas fluorescens |
| Klebsiella pneumoniae | |
Clinical Studies
In two randomized, double-masked, multicenter controlled clinical trials of 280 patients with positive cultures, subjects were dosed with IQUIX® or ofloxacin 0.3% ophthalmic solution. Dosing occurred on Days 1 through 3 every two hours while awake and 4 and 6 hours after retiring. Dosing occurred on Day 4 through treatment completion 4 times daily while awake. Clinical cure was defined as complete re-epithelialization and no progression of the infiltrate for two consecutive visits. The IQUIX® treated subjects had an approximately equal mean clinical cure rate of 80% (73% to 87%) compared to 84% (82% to 86%) for the subjects treated with ofloxacin 0.3% ophthalmic solution.
INDICATIONS AND USAGE
IQUIX® solution is indicated for the treatment of corneal ulcer caused by susceptible strains of the following bacteria:
| GRAM-POSITIVE BACTERIA: | GRAM-NEGATIVE BACTERIA: |
|---|---|
|
|
| Corynebacterium species * | Pseudomonas aeruginosa |
| Staphylococcus aureus | Serratia marcescens* |
| Staphylococcus epidermidis | |
| Streptococcus pneumoniae | |
| Viridans group streptococci* | |
CONTRAINDICATIONS
IQUIX® solution is contraindicated in patients with a history of hypersensitivity to levofloxacin, to other quinolones, or to any of the components in this medication.
WARNINGS
NOT FOR INJECTION.
IQUIX® solution should not be injected subconjunctivally, nor should it be introduced directly into the anterior chamber of the eye.
In patients receiving systemic quinolones, serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported, some following the first dose. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, angioedema (including laryngeal, pharyngeal or facial edema), airway obstruction, dyspnea, urticaria, and itching. If an allergic reaction to levofloxacin occurs, discontinue the drug. Serious acute hypersensitivity reactions may require immediate emergency treatment. Oxygen and airway management should be administered as clinically indicated.
PRECAUTIONS
General
As with other anti-infectives, prolonged use may result in overgrowth of non-susceptible organisms, induding fungi. If superinfection occurs, discontinue use and institute alternative therapy. Whenever clinical judgment dictates, the patient should be examined with the aid of magnification, such as slit-lamp biomicroscopy, and, where appropriate, fluorescein staining.
Patients should be advised not to wear contact lenses if they have signs and symptoms of corneal ulcer.
Information for Patients
Avoid contaminating the applicator tip with material from the eye, fingers or other source.
Systemic quinolones have been associated with hypersensitivity reactions, even following a single dose. Discontinue use immediately and contact your physician at the first sign of a rash or allergic reaction.
Drug Interactions
Specific drug interaction studies have not been conducted with IQUIX®. However, the systemic administration of some quinolones has been shown to elevate plasma concentrations of theophylline, interfere with the metabolism of caffeine, and enhance the effects of the oral anticoagulant warfarin and its derivatives, and has been associated with transient elevations in serum creatinine in patients receiving systemic cyclosporine concomitantly.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a long term carcinogenicity study in rats, levofloxacin exhibited no carcinogenic or tumorigenic potential following daily dietary administration for 2 years; the highest dose (100 mg/kg/day) was 100 times the highest recommended human ophthalmic dose.
Levofloxacin was not mutagenic in the following assays: Ames bacterial mutation assay (S. typhimurium and E. coli), CHO/HGPRT forward mutation assay, mouse micronucleus test, mouse dominant lethal test, rat unscheduled DNA synthesis assay, and the in vivo mouse sister chromatid exchange assay. It was positive in the in vitro chromosomal aberration (CHL cell line) and in vitro sister chromatid exchange (CHL/IU cell line) assays.
Levofloxacin caused no impairment of fertility or reproduction in rats at oral doses as high as 360 mg/kg/day, corresponding to 400 times the highest recommended human ophthalmic dose.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Levofloxacin at oral doses of 810 mg/kg/day in rats, which corresponds to approximately 1000 times the highest recommended human ophthalmic dose, caused decreased fetal body weight and increased fetal mortality.
No teratogenic effect was observed when rabbits were dosed orally as high as 50 mg/kg/day, which corresponds to approximately 60 times the highest recommended maximum human ophthalmic dose, or when dosed intravenously as high as 25 mg/kg/day, corresponding to approximately 30 times the highest recommended human ophthalmic dose.
There are, however, no adequate and well-controlled studies in pregnant women. Levofloxacin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Levofloxacin has not been measured in human milk. Based upon data from ofloxacin, it can be presumed that levofloxacin is excreted in human milk. Caution should be exercised when IQUIX® is administered to a nursing mother.
Pediatric Use
Safety and effectiveness in children below the age of six years have not been established. Oral administration of systemic quinolones has been shown to cause arthropathy in immature animals. There is no evidence that the ophthalmic administration of levofloxacin has any effect on weight bearing joints.
ADVERSE REACTIONS
The most frequently reported adverse events in the overall study population were headache and a taste disturbance following instillation. These events occurred in approximately 8–10% of patients.
Adverse events occurring in approximately 1–2% of patients included decreased/blurred vision, diarrhea, dyspepsia, fever, infection, instillation site irritation/discomfort, ocular infection, nausea, ocular pain/discomfort, and throat irritation.
Other reported ocular reactions occurring in less than 1% of patients included chemosis, corneal erosion, corneal ulcer, diplopia, floaters, hyperemia, lid edema, and lid erythema.
DOSAGE AND ADMINISTRATION
| IQUIX
levofloxacin solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Vistakon Pharmaceuticals LLC (004060273) |