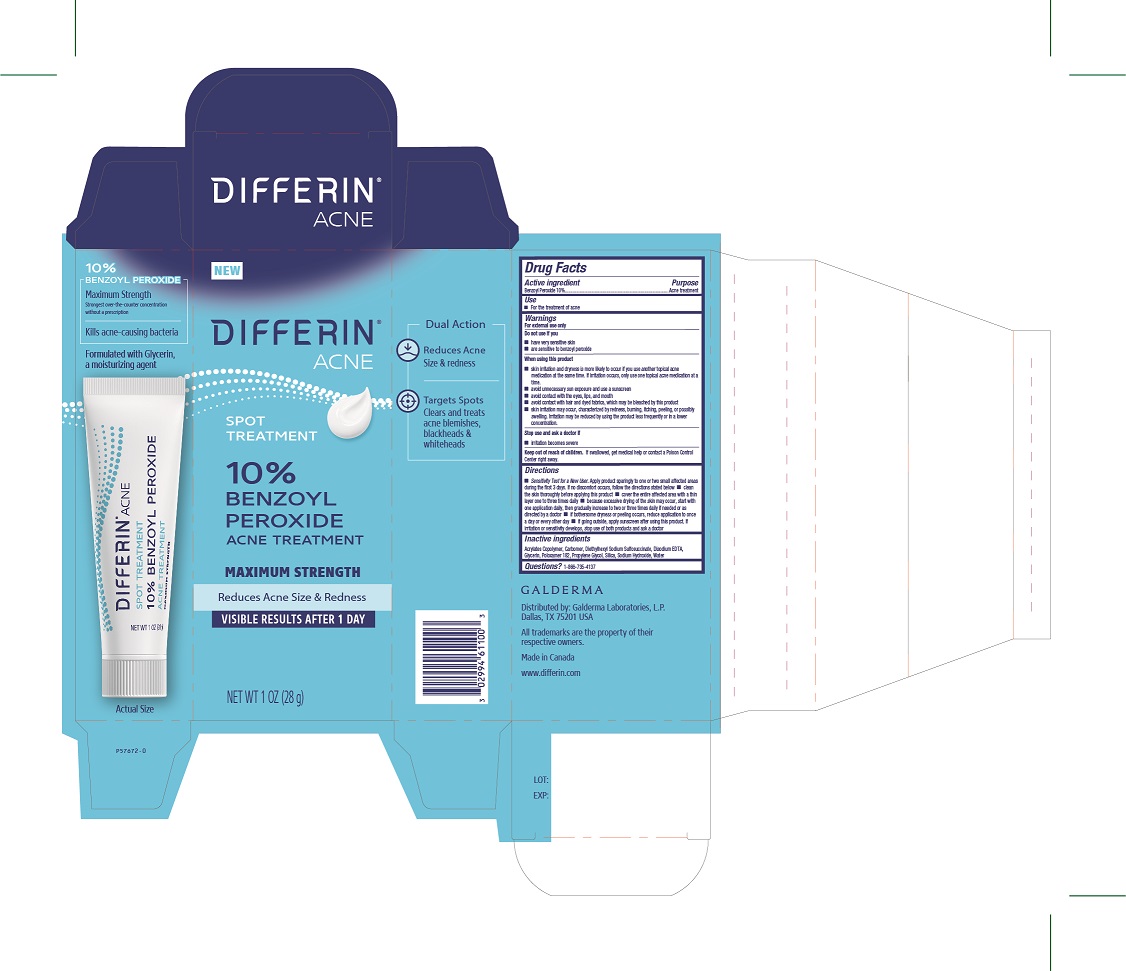
Label: DIFFERIN 10% BPO ACNE TREATMENT- benzoyl peroxide solution
- NDC Code(s): 0299-4611-00
- Packager: Galderma Laboratories, L.P.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
-
Warnings
For external use only.
Do not use if you
■ have very sensitive skin
■ are sensitive to benzoyl peroxideWhen using this product
■ skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
■ avoid unnecessary sun exposure and use a sunscreen
■ avoid contact with the eyes, lips, and mouth
■ avoid contact with hair and dyed fabrics, which may be bleached by this product
■ skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.Stop use and ask a doctor if
■ irritation becomes severe. - KEEP OUT OF REACH OF CHILDREN
-
Directions
• Sensitivity Test for a New User. Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated below ■ clean the skin thoroughly before applying this product ■ cover the entire affected area with a thin layer one to three times daily ■ because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor ■ if bothersome dryness or peeling occurs, reduce application to once a day or every other day ■ if going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor
- Inactive Ingredients
- STORAGE AND HANDLING
- Questions or comments?
- QUESTIONS
- Principle Display Panel - 1 OZ (28 g) carton
-
INGREDIENTS AND APPEARANCE
DIFFERIN 10% BPO ACNE TREATMENT
benzoyl peroxide solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0299-4611 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzoyl Peroxide (UNII: W9WZN9A0GM) (Benzoyl Peroxide - UNII:W9WZN9A0GM) Benzoyl Peroxide 10 g in 100 g Inactive Ingredients Ingredient Name Strength Butyl Acrylate/Methyl Methacrylate/Methacrylic Acid Copolymer (18000 Mw) (UNII: JZ1374NL9E) Carbomer Homopolymer, Unspecified Type (UNII: 0A5MM307FC) Docusate Sodium (UNII: F05Q2T2JA0) Edetate Disodium Anhydrous (UNII: 8NLQ36F6MM) Glycerin (UNII: PDC6A3C0OX) Poloxamer 182 (UNII: JX0HIX6OAG) Propylene Glycol (UNII: 6DC9Q167V3) Silicon Dioxide (UNII: ETJ7Z6XBU4) Sodium Hydroxide (UNII: 55X04QC32I) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0299-4611-00 1 in 1 BOX 06/06/2024 1 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/06/2024 Labeler - Galderma Laboratories, L.P. (047350186) Establishment Name Address ID/FEI Business Operations G Production Inc. 251676961 manufacture(0299-4611)