Label: ECONAZOLE NITRATE cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-5042-0 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0168-0312
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 31, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Econazole Nitrate Cream, 1% contains the antifungal agent, econazole nitrate 1%, in a water-miscible base consisting of pegoxyl 7 stearate, peglicol 5 oleate, mineral oil, benzoic acid, butylated hydroxyanisole, and purified water. The white to off-white soft cream is for topical use only.
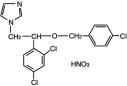
Chemically, econazole nitrate is 1-[2-{(4-chloro-phenyl)methoxy}-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole mononitrate. Its structure is as follows:
-
CLINICAL PHARMACOLOGY
After topical application to the skin of normal subjects, systemic absorption of econazole nitrate is extremely low. Although most of the applied drug remains on the skin surface, drug concentrations were found in the stratum corneum which, by far, exceeded the minimum inhibitory concentration for dermatophytes. Inhibitory concentrations were achieved in the epidermis and as deep as the middle region of the dermis. Less than 1% of the applied dose was recovered in the urine and feces.
Microbiology: Econazole nitrate has been shown to be active against most strains of the following microorganisms, bothin vitro and in clinical infections as described in theINDICATIONS AND USAGE section.
Dermatophytes Yeasts Epidermophyton floccosum Candida albicans Microsporum audouini Malassezia furfur Microsporum canis Microsporum gypseum Trichophyton mentagrophytes Trichophyton rubrum Trichophyton tonsurans Econazole nitrate exhibits broad-spectrum antifungal activity against the following organismsin vitro ,but the clinical significance of these data is unknown .
Dermatophytes Yeasts Trichophyton verrucosum Candida guillermondii Candida parapsilosis Candida tropicalis -
INDICATIONS AND USAGE
Econazole Nitrate Cream, 1% is indicated for topical application in the treatment of tinea pedis, tinea cruris, and tinea corporis caused byTrichophyton rubrum, Trichophyton mentagrophytes, Trichophyton tonsurans, Microsporum canis, Microsporum audouini, Microsporum gypseum, andEpidermophyton floccosum, in the treatment of cutaneous candidiasis, and in the treatment of tinea versicolor.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General: If a reaction suggesting sensitivity or chemical irritation should occur, use of the medication should be discontinued.
For external use only. Avoid introduction of Econazole Nitrate Cream into the eyes.
Carcinogenesis, Mutagenesis, and Impairment of Fertility: Long-term animal studies to determine carcinogenic potential have not been performed.
Pregnancy:Teratogenic effects-Pregnancy Category C : Econazole nitrate has not been shown to be teratogenic when administered orally to mice, rabbits or rats. Fetotoxic or embryotoxic effects were observed in Segment I oral studies with rats receiving 10 to 40 times the human dermal dose. Similar effects were observed in Segment II or Segment III studies with mice, rabbits and/or rats receiving oral doses 80 or 40 times the human dermal dose.
Econazole nitrate should be used in the first trimester of pregnancy only when the physician considers it essential to the welfare of the patient. The drug should be used during the second and third trimesters of pregnancy only if clearly needed.
Nursing Mothers: It is not know whether econazole nitrate is excreted in human milk. Following oral administration of econazole nitrate to lactating rats, econazole and/or metabolites were excreted in milk and were found in nursing pups. Also, in lactating rats receiving large oral doses (40 or 80 times the human dermal dose), there was a reduction in post partum viability of pups and survival to weaning; however, at these high doses, maternal toxicity was present and may have been a contributing factor. Caution should be exercised when econazole nitrate is administered to a nursing woman.
- ADVERSE REACTIONS
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Sufficient Econazole Nitrate Cream 1% should be applied to cover affected areas once daily in patients with tinea pedis, tinea cruris, tinea corporis, and tinea versicolor, and twice daily (morning and evening) in patients with cutaneous candidiasis.
Early relief of symptoms is experienced by the majority of patients and clinical improvement may be seen fairly soon after treatment is begun; however, candidal infections and tinea cruris and corporis should be treated for two weeks and tinea pedis for one month in order to reduce the possibility of recurrence. If a patient shows no clinical improvement after the treatment period, the diagnosis should be redetermined. Patients with tinea versicolor usually exhibit clinical and mycological clearing after two weeks of treatment.
-
HOW SUPPLIED
Econazole Nitrate Cream 1% is supplied as follows:
NDC 54868-5042-0 30 gram tube
Store Econazole Nitrate 1% Cream below 86°F (30°C).
E. FOUGERA & CO.
A division of Nycomed US Inc.
MELVILLE, NEW YORK 11747I2312A
R12/07
#20
Relabeling of "Additional Barcode Label" by:
Physicians Total Care, Inc.
Tulsa, OK 74146
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 30 GRAM CARTON
-
INGREDIENTS AND APPEARANCE
ECONAZOLE NITRATE
econazole nitrate creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-5042(NDC:0168-0312) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ECONAZOLE NITRATE (UNII: H438WYN10E) (ECONAZOLE - UNII:6Z1Y2V4A7M) ECONAZOLE NITRATE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) BENZOIC ACID (UNII: 8SKN0B0MIM) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-5042-0 1 in 1 CARTON 1 30 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076075 05/19/2004 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel