METHYLERGONOVINE MALEATE- methylergonovine maleate tablet
PD-Rx Pharmaceuticals, Inc.
----------
Methylergonovine Maleate Tablets, USP
DESCRIPTION
Methylergonovine Maleate is a semi-synthetic ergot alkaloid used for the prevention and control of postpartum hemorrhage.
Methylergonovine Maleate Tablets, USP is available in tablets for oral ingestion containing 0.2 mg methylergonovine maleate.
Tablets
Active ingredient: Methylergonovine maleate, USP, 0.2 mg.
Inactive ingredients: acacia, corn starch, gelatin, lactose monohydrate, methylparaben, microcrystalline cellulose, povidone, propylparaben, stearic acid, and tartaric acid.
Chemically, methylergonovine maleate is designated as ergoline-8-carboxamide, 9, 10-didehydro- N-[1-(hydroxymethyl) propyl]-6-methyl-, [8β( S)]-, (Z)-2-butenedioate (1:1) (salt). Its structural formula is:
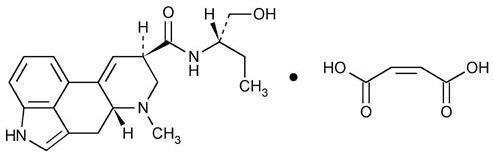
CLINICAL PHARMACOLOGY
Methylergonovine maleate acts directly on the smooth muscle of the uterus and increases the tone, rate and amplitude of rhythmic contractions. Thus, it induces a rapid and sustained tetanic uterotonic effect which shortens the third stage of labor and reduces blood loss. The onset of action after I.V. administration is immediate; after I.M. administration, 2-5 minutes, and after oral administration, 5-10 minutes.
Pharmacokinetic studies following an I.V. injection have shown that methylergonovine is rapidly distributed from plasma to peripheral tissues within 2-3 minutes or less. The bioavailability after oral administration was reported to be about 60% with no accumulation after repeated doses. During delivery, with intramuscular injection, bioavailability increased to 78%. Ergot alkaloids are mostly eliminated by hepatic metabolism and excretion, and the decrease in bioavailability following oral administration is probably a result of first-pass metabolism in the liver.
Bioavailability studies conducted in fasting healthy female volunteers have shown that oral absorption of a 0.2 mg methylergonovine tablet was fairly rapid with a mean peak plasma concentration of 3243 ± 1308 pg/mL observed at 1.12 ± 0.82 hours. For a 0.2 mg intramuscular injection, a mean peak plasma concentration of 5918 ± 1952 pg/mL was observed at 0.41 ± 0.21 hours. The extent of absorption of the tablet, based upon methylergonovine plasma concentrations, was found to be equivalent to that of the I.M. solution given orally, and the extent of oral absorption of the I.M. solution was proportional to the dose following administration of 0.1, 0.2, and 0.4 mg. When given intramuscularly, the extent of absorption of methylergonovine maleate solution was about 25% greater than the tablet. The volume of distribution (Vdss/F) of methylergonovine was calculated to be 56.1 ± 17.0 liters, and the plasma clearance (CLp/F) was calculated to be 14.4 ± 4.5 liters per hour. The plasma level decline was biphasic with a mean elimination half-life of 3.39 hours (range 1.5 to 12.7 hours). A delayed gastrointestinal absorption (Tmax about 3 hours) of methylergonovine maleate tablet might be observed in postpartum women during continuous treatment with this oxytocic agent.
INDICATIONS AND USAGE
For routine management after delivery of the placenta; postpartum atony and hemorrhage; subinvolution. Under full obstetric supervision, it may be given in the second stage of labor following delivery of the anterior shoulder.
WARNINGS
This drug should not be administered I.V. routinely because of the possibility of inducing sudden hypertensive and cerebrovascular accidents. If I.V administration is considered essential as a lifesaving measure, methylergonovine maleate should be given slowly over a period of no less than 60 seconds with careful monitoring of blood pressure. Intra-arterial or periarterial injection should be strictly avoided.
PRECAUTIONS
GENERAL
Caution should be exercised in the presence of sepsis, obliterative vascular disease, hepatic or renal involvement. Also use with caution during the second stage of labor. The necessity for manual removal of a retained placenta should occur only rarely with proper technique and adequate allowance of time for its spontaneous separation.
Drug Interactions
CYP 3A4 inhibitors (e.g., Macrolide Antibiotics and Protease Inhibitors)
There have been rare reports of serious adverse events in connection with the coadministration of certain ergot alkaloid drugs (e.g., dihydroergotamine and ergotamine) and potent CYP 3A4 inhibitors, resulting in vasospasm leading to cerebral ischemia and/or ischemia of the extremities. Although there have been no reports of such interactions with Methylergonovine alone, potent CYP 3A4 inhibitors should not be coadministered with methylergonovine. Examples of some of the more potent CYP 3A4 inhibitors include macrolide antibiotics (e.g., erythromycin, troleandomycin, clarithromycin), HIV protease or reverse transcriptase inhibitors (e.g., ritonavir, indinavir, nelfinavir, delavirdine) or azole antifungals (e.g., ketoconazole, itraconazole, voriconazole). Less potent CYP 3A4 inhibitors should be administered with caution. Less potent inhibitors include saquinavir, nefazodone, fluconazole, grapefruit juice, fluoxetine, fluvoxamine, zileuton, and clotrimazole. These lists are not exhaustive, and the prescriber should consider the effects on CYP 3A4 of other agents being considered for concomitant use with methylergonovine.
No pharmacokinetic interactions involving other cytochrome P450 isoenzymes are known.
Caution should be exercised when Methylergonovine maleate is used concurrently with other vasoconstrictors or ergot alkaloids.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies have been performed in animals to evaluate carcinogenic potential. The effect of the drug on mutagenesis or fertility has not been determined.
Pregnancy
Category C
Animal reproductive studies have not been conducted with methylergonovine maleate. It is also not known whether methylergonovine maleate can cause fetal harm or can affect reproductive capacity. Use of methylergonovine maleate is contraindicated during pregnancy because of its uterotonic effects. (See INDICATIONS AND USAGE).
Labor and Delivery
The uterotonic effect of methylergonovine maleate is utilized after delivery to assist involution and decrease hemorrhage, shortening the third stage of labor.
NURSING MOTHERS
Methylergonovine maleate may be administered orally for a maximum of 1 week postpartum to control uterine bleeding. Recommended dosage is 1 tablet (0.2 mg) 3 or 4 times daily. At this dosage level a small quantity of drug appears in mothers' milk. Caution should be exercised when methylergonovine maleate is administered to a nursing woman.
GERIATRIC USE
Clinical studies of methylergonovine maleate did not include sufficient number of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in response between the elderly and younger patients. In general dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
The most common adverse reaction is hypertension associated in several cases with seizure and/or headache. Hypotension has also been reported. Nausea and vomiting have occurred occasionally. Rarely observed reactions have included: acute myocardial infarction, transient chest pains, arterial spasm (coronary and peripheral), bradycardia, tachycardia, dyspnea, hematuria, thrombophlebitis, water intoxication, hallucinations, leg cramps, dizziness, tinnitus, nasal congestion, diarrhea, diaphoresis, palpitation, rash, and foul taste.1
There have been rare isolated reports of anaphylaxis, without a proven causal relationship to the drug product.
DRUG ABUSE AND DEPENDENCE
Methylergonovine maleate has not been associated with drug abuse or dependence of either a physical or psychological nature.
OVERDOSAGE
Symptoms of acute overdose may include: nausea, vomiting, abdominal pain, numbness, tingling of the extremities, rise in blood pressure, in severe cases followed by hypotension, respiratory depression, hypothermia, convulsions, and coma. Because reports of overdosage with Methylergonovine maleate are infrequent, the lethal dose in humans has not been established. The oral LD50 (in mg/kg) for the mouse is 187, the rat is 93, and the rabbit 4.5.2 Several cases of accidental methylergonovine Maleate injection in newborn infants have been reported, and in such cases 0.2 mg represents an overdose of great magnitude. However, recovery occurred in all but in one case following a period of respiratory depression, hypothermia, hypertonicity with jerking movements, and, in one case, a single convulsion. Also, several children 1-3 years of age have accidentally ingested up to 10 tablets (2 mg) with no apparent ill effects. A postpartum patient took 4 tablets at one time in error and reported paresthesias and clamminess as her only symptoms. Treatment of acute overdosage is symptomatic and includes the usual procedures of:
- removal of offending drug by inducing emesis, gastric lavage, catharsis, and supportive diuresis.
- maintenance of adequate pulmonary ventilation, especially if convulsions or coma develop.
- correction of hypotension with pressor drugs as needed.
- control of convulsions with standard anticonvulsant agents.
- control of peripheral vasospasm with warmth to the extremities if needed.3
DOSAGE AND ADMINISTRATION
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
HOW SUPPLIED
White, round, biconvex compressed tablets debossed with "N" on one side and "01" on the other side. Available in bottles of 3, 4, 6, 8, 9, 10, 12, 20 and 100 tablets.
REFERENCES
- Information on Adverse Reactions supplied by Medical Services Department, Novartis Pharmaceuticals, E. Hanover, N.J., based on computerized clinical reports.
- Berde, B. and Schild, H.O.: Ergot Alkaloids and Related Compounds, Springer-Verlag, New York, 1978, p. 810.
- Treatment of Acute Overdosage. Novartis Consumer Health, Inc. Rx Products. Novartis, Medical Services Department.
| METHYLERGONOVINE MALEATE
methylergonovine maleate tablet |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - PD-Rx Pharmaceuticals, Inc. (156893695) |
| Registrant - PD-Rx Pharmaceuticals, Inc. (156893695) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PD-Rx Pharmaceuticals, Inc. | 156893695 | repack(43063-329) | |
