DR. SHEFFIELD PSORIASIS MEDICATED MOISTURIZER- psoriasis medicated moisturizer lotion
Sheffield Pharmaceuticals LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
Relieves and helps prevent recurrence of
- itching
- irration
- redness
- flaking and scaling due to psoriasis and seborrheic dermatitis
Warnings
For external use only
Ask A doctor before use if condition covers a large area of the body.
When using this product avoid contact with eyes. if contact occurs, rinse throughly with water.
Stop use and ask a doctor if condition worsens or does not improve after regular use.
Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Other information
store at a controlled room temperature 68°-77°F ( 20°- 25°C)
Questions? 1-800-222-1087
Inactive ingredients
Purifed Water, Caprylic/Capric Triglyceride, Mineral Oil, Cetyl Alcohol, Butylene Glycol, Stearic Acid, Sodium Citrate, Glyceral Stearate, Triethanolamine, PEG-100 Sterate, Polysorbate 60 , Phenoxyethanol, Carbomer, Zinc PCA, Panthenol, Camellia Oleifera leaf extract , Algea Extract, Artemisia Vulgaris Extract, Dipotassium Glycyrrhizate, Disodium EDTA, Rheum Palmatum Extract, Rosa Damascena Extract, Citric Acid, Methylparaben, Propylparaben, Dimethicone, Extra Virign Olea Europaea( Olive) Fruit oil
Principal Display Panel - Carton 1.0 oz
Dr. Sheffield NDC 11527-478-64
SHEAF-FIELD TM
PSORIASIS
Salicylic Acid 2% Medicated Moisturizer
Made in the USA NET WT 1 oz. ( 28g)
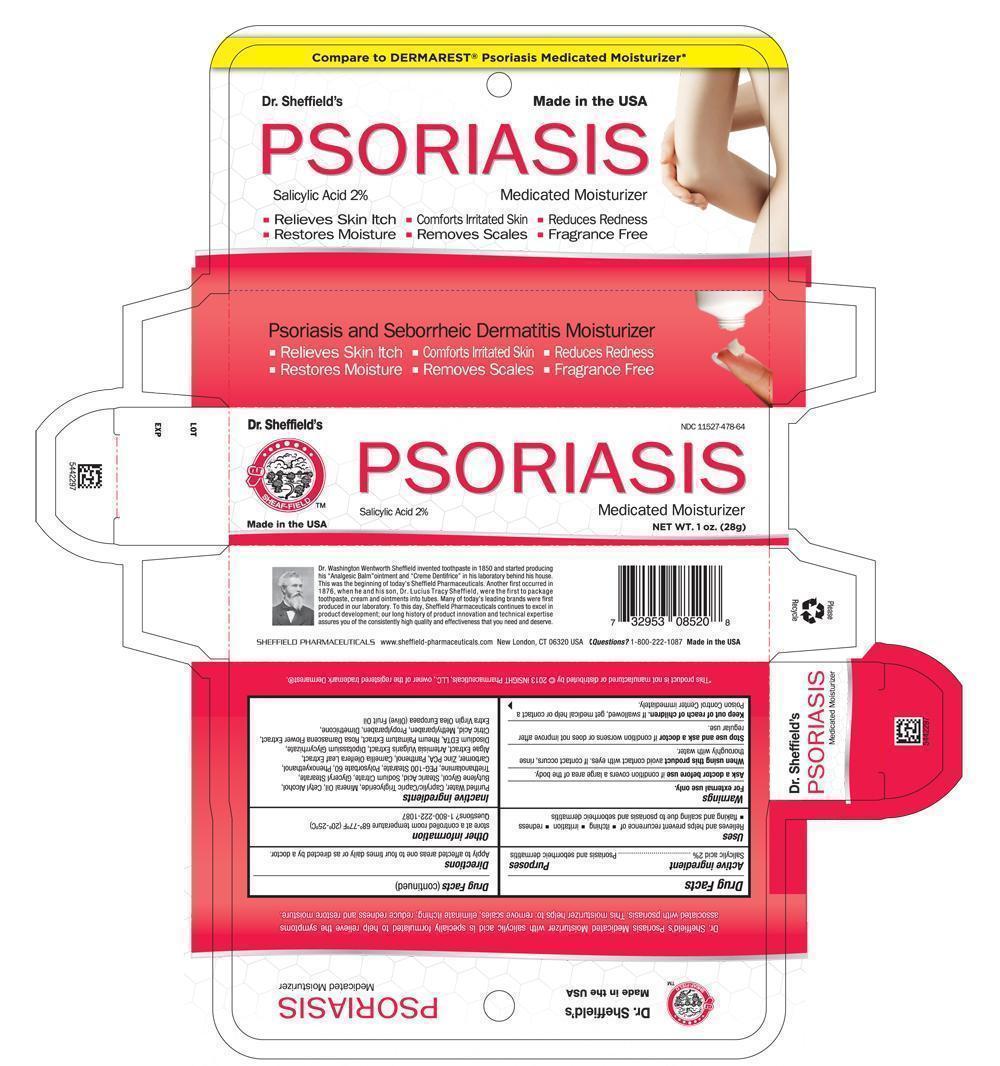
Principal Display Panel 1 oz. - Tube
Dr. Sheffield's Psoriasis Medicated Moisturizer with salicylic acid is specially formulated to help relieve the symptoms associated with psoriasis. This moisturizer helps to: remove scales, eliminate itching, reduce redness and restore moisture.
Dr. Sheffield's NDC 11527-478-64
SHEAF-FIELD TM
PSORIASIS
Salicylic Acid 2% Medicated Moisturizer
Made in the USA NET WT. 1 oz (28g)

| DR. SHEFFIELD PSORIASIS MEDICATED MOISTURIZER
psoriasis medicated moisturizer lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Sheffield Pharmaceuticals LLC (151177797) |
| Registrant - Sheffield Pharmaceuticals LLC (151177797) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sheffield Pharmaceuticals LLC | 151177797 | manufacture(11527-478) | |