Label: SODIUM CHLORIDE injection, solution, concentrate
- NDC Code(s): 0409-6657-01, 0409-6657-73, 0409-6660-01, 0409-6660-75
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
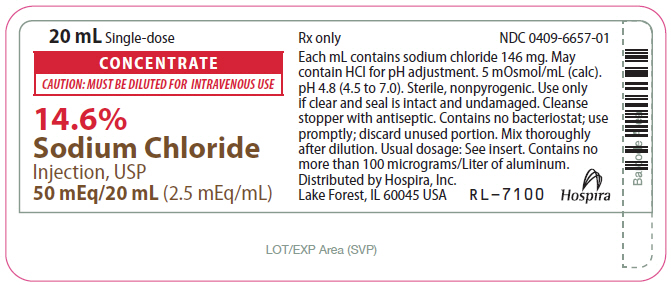
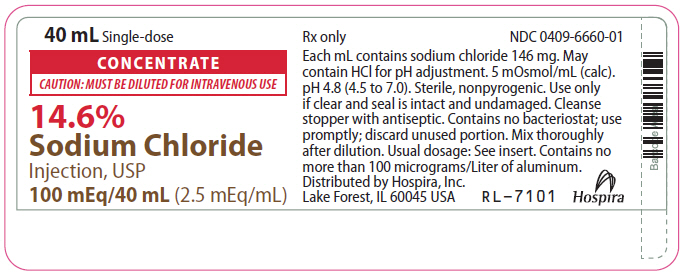
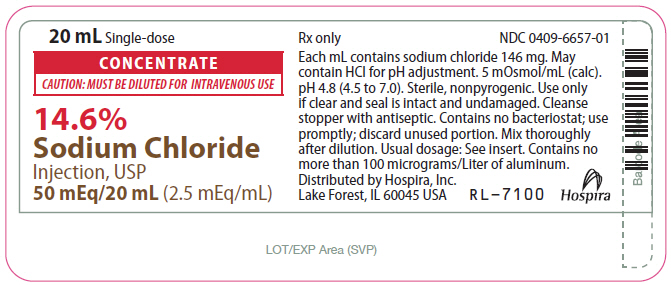
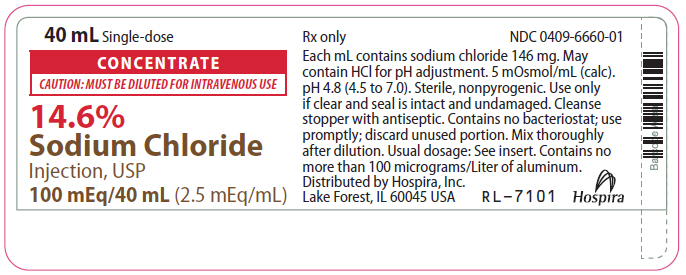
CONCENTRATE
CAUTION: MUST BE DILUTED FOR I.V. USE
14.6%
Sodium Chloride Injection, USP Rx only
50 mEq/20 mL or 100 mEq/40 mL
(2.5 mEq/mL)
Additive Solution
Concentrated Solution —For use only after dilution with compatible I.V. fluids to correct sodium deficiency when oral replacement is not feasible.
Plastic Vial
-
DESCRIPTION
14.6% Sodium Chloride Injection, USP Additive Solution is a sterile, nonpyrogenic, concentrated solution for intravenous administration ONLY AFTER DILUTION to replenish electrolytes. The preparations contain either 2.92 or 5.84 g of sodium chloride (50 or 100 mEq each of Na+ and Cl¯) in Water for Injection, USP. The solution contains no bacteriostat, antimicrobial agent or added buffer; pH 4.8 (4.5 to 7.0). May contain hydrochloric acid for pH adjustment. The osmolar concentration is 5 mOsmol/mL (calc.); specific gravity is 1.10.
Sodium Chloride, USP is chemically designated NaCl, a white crystalline compound freely soluble in water.
The semi-rigid material used for the plastic vials is fabricated from a specially formulated polyolefin. It is a copolymer of ethylene and propylene. The safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers. The container requires no vapor barrier to maintain the proper drug concentration.
-
CLINICAL PHARMACOLOGY
Sodium chloride in water dissociates to provide sodium (Na+) and chloride (Cl¯) ions. These ions are normal constituents of the body fluids (principally extracellular) and are essential for maintaining electrolyte balance.
Sodium is the principal cation of extracellular fluid. It comprises more than 90% of the total cations at its normal plasma concentration of approximately 142 mEq/liter. While the sodium ion can diffuse across cell membranes, intracellular sodium is maintained at a much lower concentration than extracellular sodium through the expenditure of energy by the cell (so called "sodium cation pump"). Loss of intracellular potassium ion is usually accompanied by an increase in intracellular sodium ion.
When serum sodium concentration is low, the secretion of antidiuretic hormone (ADH) by the pituitary is inhibited, thereby preventing water reabsorption by the distal renal tubules. On the other hand, adrenal secretion of aldosterone increases renal tubular reabsorption of sodium in an effort to re-establish normal serum sodium concentration.
Chloride (Cl¯) has an integral role in buffering action when oxygen and carbon dioxide exchange occurs in the red blood cells.
The distribution and excretion of sodium (Na+) and chloride (Cl¯) are largely under the control of the kidney which maintains a balance between intake and output.
-
INDICATIONS AND USAGE
14.6% Sodium Chloride Injection, USP Additive Solution is indicated for parenteral restoration of sodium ion in patients with restricted oral intake. Sodium replacement is specifically indicated in patients with hyponatremia or low salt syndrome. 14.6% Sodium Chloride Additive Solution may also be added to compatible carbohydrate solutions such as dextrose in water to provide electrolytes.
- CONTRAINDICATIONS
-
WARNINGS
14.6% Sodium Chloride Injection, USP is hypertonic and must be diluted prior to administration. Inadvertent direct injection or absorption of concentrated sodium chloride solution may give rise to sudden hypernatremia and such complications as cardiovascular shock, central nervous system disorders, extensive hemolysis, cortical necrosis of the kidneys and severe local tissue necrosis (if administered extravascularly).
Solutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency and in clinical states in which there exists edema with sodium retention.
In patients with diminished renal function, administration of solutions containing sodium may result in sodium retention.
The intravenous administration of this solution (after appropriate dilution) can cause fluid and/or solute overload resulting in dilution of other serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
Excessive administration of potassium free solutions may result in significant hypokalemia.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
-
PRECAUTIONS
14.6% Sodium Chloride Injection, USP Additive Solution must be diluted before infusion to avoid a sudden increase in the level of plasma sodium. Too rapid administration should be avoided.
Special caution should be used in administering sodium containing solutions to patients with severe renal impairment, cirrhosis of the liver, cardiac failure, or other edematous or sodium-retaining states.
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Caution must be exercised in the administration of parenteral fluids, especially those containing sodium ions, to patients receiving corticosteroids or corticotropin.
Do not use unless the solution is clear and seal is intact. Discard unused portion.
Pregnancy
Animal reproduction studies have not been conducted with sodium chloride. It is also not known whether sodium chloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sodium chloride should be given to a pregnant woman only if clearly needed.
Geriatric Use
An evaluation of current literature revealed no clinical experience identifying differences in response between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Pediatric Use
The safety and effectiveness of 14.6% Sodium Chloride Injection, USP Additive Solution have not been established. Its limited use in pediatric patients has been inadequate to fully define proper dosage and limitations for use.
-
ADVERSE REACTIONS
Sodium overload can occur with intravenous infusion of excessive amounts of sodium-containing solutions. (See WARNINGS and PRECAUTIONS.)
-
OVERDOSAGE
In the event of overhydration or solute overload, re-evaluate the patient and institute appropriate corrective measures. (See WARNINGS and PRECAUTIONS.)
-
DOSAGE AND ADMINISTRATION
14.6% Sodium Chloride Injection, USP Additive Solution is administered intravenously only after addition to a larger volume of fluid.
The dose, dilution and rate of injection are dependent upon the individual needs of each patient.
All or part of the contents of one or more additive containers may be added to an intravenous solution container. Concentrations of up to 5% sodium chloride have been administered.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. (See PRECAUTIONS.)
-
HOW SUPPLIED
14.6% Sodium Chloride Injection, USP Additive Solution is supplied as the following:
Unit of Sale
Concentration
Each
NDC 0409-6657-73
Tray of 25
50 mEq/20 mL
(2.5 mEq/mL)
NDC 0409-6657-01
20 mL Single-dose Plastic Fliptop Vial
NDC 0409-6660-75
Tray of 25
100 mEq/40 mL
(2.5 mEq/mL)
NDC 0409-6660-01
40 mL Single-dose Plastic Fliptop Vial
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
LAB-1099-1.0
9/2017
- PRINCIPAL DISPLAY PANEL - 20 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 20 mL Vial Tray
- PRINCIPAL DISPLAY PANEL - 40 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 40 mL Vial Tray
-
INGREDIENTS AND APPEARANCE
SODIUM CHLORIDE
sodium chloride injection, solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0409-6657 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 2.92 g in 20 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0409-6657-73 25 in 1 TRAY 10/18/2005 05/01/2018 1 NDC:0409-6657-01 20 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018897 10/18/2005 05/01/2018 SODIUM CHLORIDE
sodium chloride injection, solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0409-6660 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 5.84 g in 40 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0409-6660-75 25 in 1 TRAY 07/29/2005 1 NDC:0409-6660-01 40 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018897 07/29/2005 Labeler - Hospira, Inc. (141588017) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 093132819 ANALYSIS(0409-6657, 0409-6660) , LABEL(0409-6657, 0409-6660) , MANUFACTURE(0409-6657, 0409-6660) , PACK(0409-6657, 0409-6660) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 827731089 ANALYSIS(0409-6657, 0409-6660) Establishment Name Address ID/FEI Business Operations Pfizer Healthcare India Private Limited 860037912 ANALYSIS(0409-6657, 0409-6660) , LABEL(0409-6657, 0409-6660) , MANUFACTURE(0409-6657, 0409-6660) , PACK(0409-6657, 0409-6660)