NUVARING- etonogestrel and ethinyl estradiol insert, extended release
Organon USA Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NuvaRing safely and effectively. See full prescribing information for NuvaRing.
NuvaRing® (etonogestrel/ethinyl estradiol vaginal ring) Initial U.S. Approval: 2001 WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTSSee full prescribing information for complete boxed warning.INDICATIONS AND USAGENuvaRing is an estrogen/progestin combination hormonal contraceptive (CHC) indicated for use by women to prevent pregnancy. (1) DOSAGE AND ADMINISTRATIONOne NuvaRing is inserted in the vagina. The ring must remain in place continuously for three weeks, followed by a one-week ring-free interval. (2) DOSAGE FORMS AND STRENGTHSNuvaRing is a polymeric vaginal ring containing 11.7 mg etonogestrel and 2.7 mg ethinyl estradiol, which releases on average 0.12 mg/day of etonogestrel and 0.015 mg/day of ethinyl estradiol. (3) CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions (≥2%) in clinical trials were: vaginitis, headache (including migraine), mood changes (e.g., depression, mood swings, mood altered, depressed mood, affect lability), device-related events (e.g., expulsion/discomfort/foreign body sensation), nausea/vomiting, vaginal discharge, increased weight, vaginal discomfort, breast pain/discomfort/tenderness, dysmenorrhea, abdominal pain, acne, and decreased libido. (6) To report SUSPECTED ADVERSE REACTIONS, contact Merck, Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSDrugs or herbal products that induce certain enzymes, such as CYP3A4, may decrease the effectiveness of CHCs or increase breakthrough bleeding. Counsel patients to use a back-up or alternative method of contraception when enzyme inducers are used with CHCs. (7) See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 1/2020 |
FULL PRESCRIBING INFORMATION
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
Cigarette smoking increases the risk of serious cardiovascular events from combination hormonal contraceptive (CHC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, CHCs, including NuvaRing, should not be used by women who are over 35 years of age and smoke. [See Contraindications (4).]
1 INDICATIONS AND USAGE
FOR VAGINAL USE ONLY
NuvaRing® is indicated for use by females of reproductive age to prevent pregnancy.
2 DOSAGE AND ADMINISTRATION
2.1 How to Use NuvaRing
To achieve maximum contraceptive effectiveness, NuvaRing must be used as directed [see Dosage and Administration (2.2)]. One NuvaRing is inserted in the vagina. The ring is to remain in place continuously for three weeks. It is removed for a one-week break, during which a withdrawal bleed usually occurs. A new ring is inserted one week after the last ring was removed.
The user can choose the insertion position that is most comfortable to her, for example, standing with one leg up, squatting, or lying down. The ring is to be compressed and inserted into the vagina. An optional alternative is to insert the ring using the applicator for NuvaRing [see Applicator for NuvaRing Instructions for Use]. The exact position of NuvaRing inside the vagina is not critical for its function. The vaginal ring must be inserted on the appropriate day and left in place for three consecutive weeks. This means that the ring should be removed three weeks later on the same day of the week as it was inserted and at about the same time.
NuvaRing can be removed by hooking the index finger under the forward rim or by grasping the rim between the index and middle finger and pulling it out. The used ring should be placed in the sachet (foil pouch) and discarded in a waste receptacle out of the reach of children and pets (do not flush in toilet).
After a one-week break, during which a withdrawal bleed usually occurs, a new ring is inserted on the same day of the week as it was inserted in the previous cycle. The withdrawal bleed usually starts on Day 2-3 after removal of the ring and may not have finished before the next ring is inserted. In order to maintain contraceptive effectiveness, the new ring must be inserted exactly one week after the previous one was removed even if menstrual bleeding has not finished.
2.2 How to Start Using NuvaRing
IMPORTANT: Consider the possibility of ovulation and conception prior to the first use of NuvaRing.
No Hormonal Contraceptive Use in the Preceding Cycle:
The woman should insert NuvaRing on the first day of her menstrual bleeding. NuvaRing may also be started on Days 2-5 of the woman's cycle, but in this case a barrier method, such as male condoms with spermicide, should be used for the first seven days of NuvaRing use in the first cycle.
Changing From a CHC:
The woman may switch from her previous CHC on any day, but at the latest on the day following the usual hormone-free interval, if she has been using her hormonal method consistently and correctly, or if it is reasonably certain that she is not pregnant.
Changing From a Progestin-Only Method (progestin-only pill [POP], Implant, or Injection or a Progestin-Releasing Intrauterine System [IUS]):
The woman may switch from the POP on any day; instruct her to start using NuvaRing on the day after she took her last POP. She should switch from an implant or the IUS on the day of its removal, and from an injectable on the day when the next injection would be due. In all of these cases, the woman should use an additional barrier method such as a male condom with spermicide, for the first seven days.
Use After Abortion or Miscarriage:
The woman may start using NuvaRing within the first five days following a complete first trimester abortion or miscarriage, and she does not need to use an additional method of contraception. If use of NuvaRing is not started within five days following a first trimester abortion or miscarriage, the woman should follow the instructions for "No Hormonal Contraceptive Use in the Preceding Cycle." In the meantime, she should be advised to use a non-hormonal contraceptive method.
Start NuvaRing no earlier than four weeks after a second trimester abortion or miscarriage, due to the increased risk of thromboembolism. [See Contraindications (4) and Warnings and Precautions (5.1).]
Following Childbirth:
The use of NuvaRing may be initiated no sooner than four weeks postpartum in women who elect not to breastfeed, due to the increased risk of thromboembolism in the postpartum period. [See Contraindications (4) and Warnings and Precautions (5.1).]
Advise women who are breastfeeding not to use NuvaRing but to use other forms of contraception until the child is weaned.
If a woman begins using NuvaRing postpartum, instruct her to use an additional method of contraception, such as male condoms with spermicide, for the first seven days. If she has not yet had a period, consider the possibility of ovulation and conception occurring prior to initiation of NuvaRing.
2.3 Deviations from the Recommended Regimen
To prevent loss of contraceptive efficacy, advise women not to deviate from the recommended regimen. NuvaRing should be left in the vagina for a continuous period of three weeks. Advise women to regularly check for the presence of NuvaRing in the vagina (for example, before and after intercourse).
Inadvertent Removal or Expulsion:
NuvaRing can be accidentally expelled, for example, while removing a tampon, during intercourse, or with straining during a bowel movement. NuvaRing should be left in the vagina for a continuous period of three weeks. If the ring is accidentally expelled and is left outside of the vagina for less than three hours, contraceptive efficacy is not reduced. NuvaRing can be rinsed with cool to lukewarm (not hot) water and reinserted as soon as possible, but at the latest within three hours. If NuvaRing is lost, a new vaginal ring should be inserted and the regimen should be continued without alteration.
If NuvaRing is out of the vagina for more than three continuous hours:
During Weeks 1 and 2: Contraceptive efficacy may be reduced. The woman should reinsert the ring as soon as she remembers. A barrier method such as male condoms with spermicides must be used until the ring has been used continuously for seven days.
During Week 3: The woman should discard that ring. One of the following two options should be chosen:
- Insert a new ring immediately. Inserting a new ring will start the next three-week use period. The woman may not experience a withdrawal bleed from her previous cycle. However, breakthrough spotting or bleeding may occur.
- Insert a new ring no later than seven days from the time the previous ring was removed or expelled, during which time she may have a withdrawal bleed. This option should only be chosen if the ring was used continuously for at least seven days prior to inadvertent removal/expulsion.
In either case, a barrier method such as male condoms with spermicides must be used until the new ring has been used continuously for seven days.
If NuvaRing was out of the vagina for an unknown amount of time, the possibility of pregnancy should be considered. A pregnancy test should be performed prior to inserting a new ring.
Prolonged Ring-Free Interval:
If the ring-free interval has been extended beyond one week, consider the possibility of pregnancy, and an additional method of contraception, such as male condoms with spermicide, MUST be used until NuvaRing has been used continuously for seven days.
Prolonged Use of NuvaRing:
If NuvaRing has been left in place for up to one extra week (i.e., up to four weeks total), the woman will remain protected. NuvaRing should be removed and the woman should insert a new ring after a one-week ring-free interval.
If NuvaRing has been left in place for longer than four weeks, instruct the woman to remove the ring, and rule out pregnancy. If pregnancy is ruled out, NuvaRing may be restarted, and an additional method of contraception, such as male condoms with spermicide, MUST be used until a new NuvaRing has been used continuously for seven days.
Ring Breakage:
There have been reported cases of NuvaRing disconnecting at the weld joint. This is not expected to affect the contraceptive effectiveness of NuvaRing. In the event of a disconnected ring, vaginal discomfort or expulsion (slipping out) is more likely to occur. Vaginal injury associated with ring breakage has been reported [see Adverse Reactions (6.2)].
If a woman discovers that her NuvaRing has disconnected, she should discard the ring and replace it with a new ring.
2.4 In the Event of a Missed Menstrual Period
- If the woman has not adhered to the prescribed regimen (NuvaRing has been out of the vagina for more than three hours or the preceding ring-free interval was extended beyond one week), consider the possibility of pregnancy at the time of the first missed period and discontinue NuvaRing use if pregnancy is confirmed.
- If the woman has adhered to the prescribed regimen and misses two consecutive periods, rule out pregnancy.
- If the woman has retained one NuvaRing for longer than four weeks, rule out pregnancy.
2.5 Use with Other Vaginal Products
NuvaRing may interfere with the correct placement and position of certain female barrier methods such as a diaphragm, cervical cap or female condom. These methods are not recommended as back-up methods with NuvaRing use.
Pharmacokinetic data show that the use of tampons has no effect on the systemic absorption of the hormones released by NuvaRing.
3 DOSAGE FORMS AND STRENGTHS
NuvaRing (etonogestrel/ethinyl estradiol vaginal ring) is a non-biodegradable, flexible, transparent, colorless to almost colorless, combination contraceptive vaginal ring, with an outer diameter of 54 mm and a cross-sectional diameter of 4 mm. It is made of ethylene vinylacetate copolymers and magnesium stearate, and contains 11.7 mg etonogestrel and 2.7 mg ethinyl estradiol. When placed in the vagina, each ring releases on average 0.120 mg/day of etonogestrel and 0.015 mg/day of ethinyl estradiol over a three-week period of use. NuvaRing is not made with natural rubber latex.
4 CONTRAINDICATIONS
Do not prescribe NuvaRing to women who are known to have or use the following:
- A high risk of arterial or venous thrombotic diseases. Examples include women who are known to:
- Smoke, if over age 35 [see Boxed Warning and Warnings and Precautions (5.1)]
- Have deep vein thrombosis or pulmonary embolism, now or in the past [see Warnings and Precautions (5.1)]
- Have cerebrovascular disease [see Warnings and Precautions (5.1)]
- Have coronary artery disease [see Warnings and Precautions (5.1)]
- Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation) [see Warnings and Precautions (5.1)]
- Have inherited or acquired hypercoagulopathies [see Warnings and Precautions (5.1)]
- Have uncontrolled hypertension [see Warnings and Precautions (5.5)]
- Have diabetes mellitus with vascular disease [see Warnings and Precautions (5.9)]
- Have headaches with focal neurological symptoms or migraine headaches with aura [see Warnings and Precautions (5.10)]
- Women over age 35 with any migraine headaches [see Warnings and Precautions (5.10)]
- Liver tumors, benign or malignant or liver disease [see Warnings and Precautions (5.3) and Use in Specific Populations (8.6)]
- Undiagnosed abnormal uterine bleeding [see Warnings and Precautions (5.11)]
- Pregnancy, because there is no reason to use CHCs during pregnancy [see Use in Specific Populations (8.1)]
- Breast cancer or other estrogen- or progestin-sensitive cancer, now or in the past [see Warnings and Precautions (5.14)]
- Hypersensitivity reactions, including anaphylaxis and angioedema, to any of the components of NuvaRing [see Warnings and Precautions (5.6) and Adverse Reactions (6)]
- Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to potential for ALT elevations [see Warnings and Precautions (5.4)]
5 WARNINGS AND PRECAUTIONS
5.1 Thromboembolic Disorders and Other Vascular Problems
Stop NuvaRing use if an arterial thrombotic or venous thromboembolic event (VTE) occurs. Stop NuvaRing use if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately. [See Adverse Reactions (6).]
If feasible, stop NuvaRing at least four weeks before and through two weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism, and during and following prolonged immobilization.
Start NuvaRing no earlier than 4 weeks after delivery, in women who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the risk of ovulation increases after the third postpartum week.
The use of CHCs increases the risk of VTE. Known risk factors for VTE include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of CHCs [see Contraindications (4)].
Two epidemiologic studies1, 2, 3 that assessed the risk of VTE associated with the use of NuvaRing are described below.
In these studies, which were required or sponsored by regulatory agencies, NuvaRing users had a risk of VTE similar to Combined Oral Contraceptives (COCs) users (see Table 1 for adjusted hazard ratios). A large prospective, observational study, the Transatlantic Active Surveillance on Cardiovascular Safety of NuvaRing (TASC), investigated the risk of VTE for new users, and women who were switching to or restarting NuvaRing or COCs in a population that is representative of routine clinical users. The women were followed for 24 to 48 months. The results showed a similar risk of VTE among NuvaRing users (VTE incidence 8.3 per 10,000 WY) and women using COCs (VTE incidence 9.2 per 10,000 WY). For women using COCs that did not contain the progestins desogestrel (DSG) or gestodene (GSD), VTE incidence was 8.9 per 10,000 WY.
A retrospective cohort study using data from 4 health plans in the US (FDA-funded Study in Kaiser Permanente and Medicaid databases) showed the VTE incidence for new users of NuvaRing to be 11.4 events per 10,000 WY, for new users of a levonorgestrel (LNG)-containing COC 9.2 events per 10,000 WY, and for users of other COCs available during the course of the study1 8.2 events per 10,000 WY.
| Epidemiologic Study (Author, Year of Publication) Population Studied | Comparator Product(s) | Hazard Ratios (HR) (95% CI) |
|---|---|---|
|
||
| TASC (Dinger, 2012) Initiators, including new users, switchers and restarters |
All COCs available during the course of the study * COCs available excluding DSG- or GSD -containing OCs |
HR†: 0.8 (0.5-1.5) HR†: 0.8 (0.4-1.7) |
| FDA-funded Study in Kaiser Permanente and Medicaid databases (Sidney, 2011) | ||
| First use of a combined hormonal contraceptive (CHC) during the study period | COCs available during the course of the study‡ | HR§: 1.1 (0.6-2.2) |
| LNG/0.03 mg ethinyl estradiol | HR§: 1.0 (0.5-2.0) |
|
An increased risk of thromboembolic and thrombotic disease associated with the use of CHCs is well-established. Although the absolute VTE rates are increased for users of CHCs compared to non-users, the rates associated with pregnancy are even greater, especially during the post-partum period (see Figure 1).
The frequency of VTE in women using CHCs has been estimated to be 3 to 12 cases per 10,000 women-years.
The risk of VTE is highest during the first year of CHC use and after restarting a CHC following a break of at least four weeks. The risk of VTE due to CHCs gradually disappears after use is discontinued.
Figure 1 shows the risk of developing a VTE for women who are not pregnant and do not use CHCs, for women who use CHCs, for pregnant women, and for women in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 women who are not pregnant and do not use CHCs are followed for one year, between 1 and 5 of these women will develop a VTE.
Figure 1: Likelihood of Developing a VTE
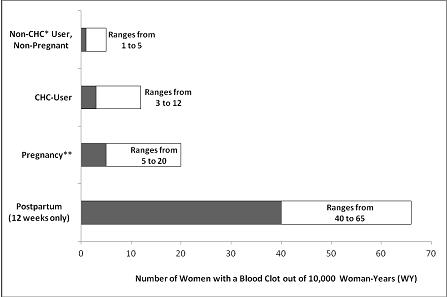
*CHC=combination hormonal contraception
**Pregnancy data based on actual duration of pregnancy in the reference studies. Based on a model assumption that pregnancy duration is nine months, the rate is 7 to 27 per 10,000 WY.
Several epidemiology studies indicate that third generation oral contraceptives, including those containing desogestrel (etonogestrel, the progestin in NuvaRing, is the biologically active metabolite of desogestrel), may be associated with a higher risk of VTE than oral contraceptives containing other progestins. Some of these studies indicate an approximate two-fold increased risk. However, data from other studies have not shown this two-fold increase in risk.
Use of CHCs also increases the risk of arterial thromboses such as strokes and myocardial infarctions, especially in women with other risk factors for these events. CHCs have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes). In general, the risk is greatest among older (>35 years of age), hypertensive women who also smoke.
Use NuvaRing with caution in women with cardiovascular disease risk factors.
- 1
- Includes low-dose COCs containing the following progestins: norgestimate, norethindrone, or levonorgestrel.
5.2 Toxic Shock Syndrome (TSS)
Cases of TSS have been reported by NuvaRing users. TSS has been associated with tampons and certain barrier contraceptives, and, in some cases the NuvaRing users were also using tampons. A causal relationship between the use of NuvaRing and TSS has not been established. If a patient exhibits signs or symptoms of TSS, consider the possibility of this diagnosis and initiate appropriate medical evaluation and treatment.
5.3 Liver Disease
Impaired Liver Function
Do not use NuvaRing in women with liver disease such as acute viral hepatitis or severe (decompensated) cirrhosis of the liver [see Contraindications (4)]. Acute or chronic disturbances of liver function may necessitate the discontinuation of CHC use until markers of liver function return to normal and CHC causation has been excluded [see Use in Specific Populations (8.6)]. Discontinue NuvaRing use if jaundice develops.
Liver Tumors
NuvaRing is contraindicated in women with benign and malignant liver tumors [see Contraindications (4)]. Hepatic adenomas are associated with CHC use. An estimate of the attributable risk is 3.3 cases per 100,000 CHC users. Rupture of hepatic adenomas may cause death through intra-abdominal hemorrhage.
Studies have shown an increased risk of developing hepatocellular carcinoma in long term (>8 years) CHC users. However, the attributable risk of liver cancers in CHC users is less than one case per million users.
5.4 Risk of Liver Enzyme Elevations with Concomitant Hepatitis C Treatment
During clinical trials with the Hepatitis C combination drug regimen that contains ombitasvir/paritaprevir/ritonavir, with and without dasabuvir, ALT elevations greater than 5 times the upper limit of normal (ULN), including some cases greater than 20 times the ULN, were significantly more frequent in women using ethinyl estradiol-containing medications, such as CHCs. Discontinue NuvaRing prior to starting therapy with the combination drug regimen ombitasvir/paritaprevir/ritonavir, with or without dasabuvir [see Contraindications (4)]. NuvaRing can be restarted approximately 2 weeks following completion of treatment with the Hepatitis C combination drug regimen.
5.5 High Blood Pressure
NuvaRing is contraindicated in women with uncontrolled hypertension or hypertension with vascular disease [see Contraindications (4)]. For women with well-controlled hypertension, monitor blood pressure and stop NuvaRing use if blood pressure rises significantly.
An increase in blood pressure has been reported in women using CHCs and this increase is more likely in older women and with extended duration of use. The incidence of hypertension increases with increasing concentrations of progestin.
5.6 Hypersensitivity Reactions
Hypersensitivity reactions of anaphylaxis and angioedema have been reported during use of NuvaRing. If anaphylaxis and/or angioedema is suspected, NuvaRing should be discontinued and appropriate treatment administered. [See Contraindications (4).]
5.7 Vaginal Use
NuvaRing may not be suitable for women with conditions that make the vagina more susceptible to vaginal irritation or ulceration. Vaginal/cervical erosion or ulceration in women using NuvaRing has been reported. In some cases, the ring adhered to vaginal tissue, necessitating removal by a healthcare provider and in some instances (i.e., when the tissue had grown over the ring), removal was achieved by cutting the ring without incising the overlying vaginal tissue.
Some women are aware of the ring on occasion during the 21 days of use or during intercourse, and sexual partners may feel NuvaRing in the vagina.
5.8 Gallbladder Disease
Studies suggest a small increased relative risk of developing gallbladder disease among CHC users. Use of CHCs may also worsen existing gallbladder disease.
A past history of CHC-related cholestasis predicts an increased risk with subsequent CHC use. Women with a history of pregnancy-related cholestasis may be at an increased risk for CHC-related cholestasis.
5.9 Carbohydrate and Lipid Metabolic Effects
Carefully monitor prediabetic and diabetic women who are using NuvaRing. CHCs may decrease glucose tolerance.
Consider alternative contraception for women with uncontrolled dyslipidemia. Some women will have adverse lipid changes while on CHCs.
Women with hypertriglyceridemia, or a family history thereof, may be at an increased risk of pancreatitis when using CHCs.
5.10 Headache
If a woman using NuvaRing develops new headaches that are recurrent, persistent, or severe, evaluate the cause and discontinue NuvaRing if indicated.
Consider discontinuation of NuvaRing in the case of an increased frequency or severity of migraine during CHC use (which may be prodromal of a cerebrovascular event) [see Contraindications (4)].
5.11 Bleeding Irregularities and Amenorrhea
Unscheduled Bleeding and Spotting
Unscheduled bleeding (breakthrough or intracyclic) bleeding and spotting sometimes occur in women using CHCs, especially during the first three months of use. If bleeding persists or occurs after previously regular cycles, check for causes such as pregnancy or malignancy. If pathology and pregnancy are excluded, bleeding irregularities may resolve over time or with a change to a different CHC.
Bleeding patterns were evaluated in three large clinical studies. In the North American study (US and Canada, N=1,177), the percentages of subjects with breakthrough bleeding/spotting ranged from 7.2% to 11.7% during cycles 1-13. In the two non-US studies, the percentages of subjects with breakthrough bleeding/spotting ranged from 2.6% to 6.4% (Europe, N=1,145) and from 2.0% to 8.7% (Europe, Brazil, Chile, N=512).
Amenorrhea and Oligomenorrhea
If scheduled (withdrawal) bleeding does not occur, consider the possibility of pregnancy. If the patient has not adhered to the prescribed dosing schedule, consider the possibility of pregnancy at the time of the first missed period and take appropriate diagnostic measures.
Occasional missed periods may occur with the appropriate use of NuvaRing. In the clinical studies, the percent of women who did not have withdrawal bleeding in a given cycle ranged from 0.3% to 3.8%.
If the patient has adhered to the prescribed regimen and misses two consecutive periods, rule out pregnancy.
Some women may experience amenorrhea or oligomenorrhea after discontinuing CHC use, especially when such a condition was pre-existent.
5.12 Inadvertent Urinary Bladder Insertion
There have been reports of inadvertent insertions of NuvaRing into the urinary bladder, which required cystoscopic removal. Assess for ring insertion into the urinary bladder in NuvaRing users who present with persistent urinary symptoms and are unable to locate the ring.
5.13 Depression
Carefully observe women with a history of depression and discontinue NuvaRing use if depression recurs to a serious degree.
5.14 Carcinoma of the Breasts and Cervix
NuvaRing is contraindicated in women who currently have or have had breast cancer because breast cancer is a hormonally-sensitive tumor [see Contraindications (4)].
There is substantial evidence that CHCs do not increase the incidence of breast cancer. Although some past studies have suggested that CHCs might increase the incidence of breast cancer, more recent studies have not confirmed such findings.
Some studies suggest that CHCs are associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. However, there is controversy about the extent to which these findings may be due to differences in sexual behavior and other factors.
5.15 Effect on Binding Globulins
The estrogen component of CHCs may raise the serum concentrations of thyroxine-binding globulin, sex hormone-binding globulin, and cortisol-binding globulin. The dose of replacement thyroid hormones or cortisol therapy may need to be increased.
5.16 Monitoring
A woman who is using NuvaRing should have a yearly visit with her healthcare provider for a blood pressure check and for other indicated healthcare.
6 ADVERSE REACTIONS
The following serious adverse reactions with the use of CHCs are discussed elsewhere in the labeling.
- Serious cardiovascular events and stroke [see Boxed Warning and Warnings and Precautions (5.1)]
- Vascular events [see Warnings and Precautions (5.1)]
- Liver disease [see Warnings and Precautions (5.3)]
Adverse reactions commonly reported by CHC users are:
- Irregular uterine bleeding
- Nausea
- Breast tenderness
- Headache
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Trials with a duration of 6 to 13 28-day cycles provided safety data. In total, 2,501 women, aged 18 to 41 contributed 24,520 cycles of exposure.
Common Adverse Reactions (≥ 2%): vaginitis (13.8%), headache (including migraine) (11.2%), mood changes (e.g., depression, mood swings, mood altered, depressed mood, affect lability) (6.4%), device-related events (e.g., expulsion/discomfort/foreign body sensation) (6.3%), nausea/vomiting (5.9%), vaginal discharge (5.7%), increased weight (4.9%), vaginal discomfort (4.0%), breast pain/discomfort/tenderness (3.8%), dysmenorrhea (3.5%), abdominal pain (3.2%), acne (2.4%), and decreased libido (2.0%).
Adverse Reactions (≥ 1%) Leading to Study Discontinuation: 13.0% of the women discontinued from the clinical trials due to an adverse reaction; the most common adverse reactions leading to discontinuation were device-related events (2.7%), mood changes (1.7%), headache (including migraine) (1.5%) and vaginal symptoms (1.2%).
Serious Adverse Reactions: deep vein thrombosis [see Warnings and Precautions (5.1)], anxiety, cholelithiasis, and vomiting.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of NuvaRing. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders: hypersensitivity reactions, including anaphylaxis and angioedema
Nervous system disorders: stroke/cerebrovascular accident
Vascular disorders: arterial events (including arterial thromboembolism and myocardial infarction), aggravation of varicose veins
Skin and subcutaneous tissue disorders: urticaria, chloasma
Reproductive system and breast disorders: penile disorders, including local reactions on penis (in male partners of women using NuvaRing), galactorrhea
General Disorders and Administration Site Conditions: device breakage (including with concomitant use of intravaginal antimycotic, antibiotic, and lubricant products)
Injury, poisoning and procedural complications: vaginal injury (including associated pain, discomfort, and bleeding) associated with ring breakage
7 DRUG INTERACTIONS
Consult the labeling of all concurrently-used drugs to obtain further information about interactions with hormonal contraceptives or the potential for enzyme alterations.
7.1 Effects of Other Drugs on CHCs
Substances decreasing the plasma concentrations of CHCs and potentially diminishing the effectiveness of CHCs
Drugs or herbal products that induce certain enzymes, including cytochrome P450 3A4 (CYP3A4), may decrease the plasma concentrations of CHCs and potentially diminish the effectiveness of CHCs or increase breakthrough bleeding. Some drugs or herbal products that may decrease the effectiveness of hormonal contraceptives include: phenytoin, barbiturates, carbamazepine, bosentan, felbamate, griseofulvin, oxcarbazepine, rifampicin, topiramate, rifabutin, rufinamide, aprepitant, and products containing St. John's wort. Interactions between CHCs and other drugs may lead to breakthrough bleeding and/or contraceptive failure.
Counsel women to use an alternative non-hormonal method of contraception or a back-up method when enzyme inducers are used with NuvaRing, and to continue back-up non-hormonal contraception for 28 days after discontinuing the enzyme inducer to ensure contraceptive reliability.
Note: NuvaRing may interfere with the correct placement and position of certain female barrier methods such as a diaphragm or female condom. These methods are not recommended as back-up methods with NuvaRing use [see Dosage and Administration (2.5)].
The serum concentrations of etonogestrel and ethinyl estradiol were not affected by concomitant administration of oral amoxicillin or doxycycline in standard dosages during 10 days of antibiotic treatment. The effects of other antibiotics on etonogestrel or ethinyl estradiol concentrations have not been evaluated.
Substances increasing the plasma concentrations of CHCs
Co-administration of atorvastatin and certain CHCs containing ethinyl estradiol increase AUC values for ethinyl estradiol by approximately 20-25%. Ascorbic acid and acetaminophen may increase plasma ethinyl estradiol concentrations, possibly by inhibition of conjugation. Concomitant administration of strong or moderate CYP3A4 inhibitors such as itraconazole, voriconazole, fluconazole, grapefruit juice, or ketoconazole may increase plasma estrogen and/or progestin concentrations. Co-administration of vaginal miconazole nitrate and NuvaRing increases the serum concentrations of etonogestrel and ethinyl estradiol by up to 40% [see Clinical Pharmacology (12.3)].
Human immunodeficiency virus (HIV) / Hepatitis C Virus (HCV) protease inhibitors and non-nucleoside reverse transcriptase inhibitors
Significant changes in the plasma concentrations of the estrogen and /or progestin have been noted in some cases of co-administration with HIV protease inhibitors (decrease [e.g., nelfinavir, ritonavir, darunavir/ritonavir, (fos)amprenavir/ritonavir, lopinavir/ritonavir, and tipranavir/ritonavir] or increase [e.g., indinavir and atazanavir/ritonavir]) /HCV protease inhibitors (decrease [e.g., boceprevir and telaprevir]) or with non-nucleoside reverse transcriptase inhibitors (decrease [e.g., efavirenz, nevirapine] or increase [e.g., etravirine]). These changes may be clinically relevant in some cases.
7.2 Effects of CHCs on Other Drugs
CHCs containing ethinyl estradiol may inhibit the metabolism of other compounds (e.g., cyclosporine, prednisolone, theophylline, tizanidine, and voriconazole) and increase their plasma concentrations. CHCs have been shown to decrease plasma concentrations of acetaminophen, clofibric acid, morphine, salicylic acid and temazepam. A significant decrease in the plasma concentrations of lamotrigine has been shown, likely due to induction of lamotrigine glucuronidation. This may reduce seizure control; therefore, dosage adjustments of lamotrigine may be necessary.
Women on thyroid hormone replacement therapy may need increased doses of thyroid hormone because serum concentrations of thyroid-binding globulin increase with use of CHCs.
7.3 Concomitant Use with HCV Combination Therapy – Liver Enzyme Elevation
Do not co-administer NuvaRing with HCV drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to potential for ALT elevations [see Warnings and Precautions (5.4)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
NuvaRing is contraindicated during pregnancy because there is no need for pregnancy prevention in a woman who is already pregnant. Epidemiologic studies and meta-analyses have not shown an increased risk of genital or non-genital birth defects (including cardiac anomalies and limb-reduction defects) following maternal exposure to low dose CHCs prior to conception or during early pregnancy. No adverse developmental outcomes were observed in pregnant rats and rabbits with the administration of etonogestrel during organogenesis at doses approximately 300 times the anticipated daily vaginal human dose (~0.002 mg/kg/day).
No adverse developmental outcomes were observed in pregnant rats and rabbits with the co-administration of the combination desogestrel/ethinyl estradiol during organogenesis at desogestrel/ethinyl estradiol doses at least 2/5 times, respectively, the anticipated daily vaginal human dose (~0.002 desogestrel/0.00025 ethinyl estradiol mg/kg/day).
Discontinue NuvaRing use if pregnancy is confirmed.
Data
Animal Data
In rats and rabbits at dosages up to 300 times the anticipated dose, etonogestrel is neither embryotoxic nor teratogenic. Co-administration of a maternally toxic dose of desogestrel/ethinyl estradiol to pregnant rats was associated with embryolethality and wavy ribs at a desogestrel/ethinyl estradiol dose that was 40/130 times, respectively, the anticipated vaginal human dose (0.002 desogestrel/0.00025 ethinyl estradiol mg/kg/day). No adverse embryofetal effects were observed when the combination was administered to pregnant rats at a desogestrel/ethinyl estradiol dose that was 4/13 times, respectively, the anticipated vaginal human dose. When desogestrel/ethinyl estradiol was given to pregnant rabbits, pre-implantation loss was observed at a desogestrel/ethinyl estradiol dose that was 3/10 times, respectively, the anticipated vaginal human dose. No adverse embryofetal effects were observed when the combination was administered to pregnant rabbits at a desogestrel/ethinyl estradiol dose that was 2/5 times the anticipated vaginal human dose.
8.2 Lactation
Risk Summary
Small amounts of contraceptive steroids and/or metabolites, including etonogestrel and ethinyl estradiol are transferred to human milk. Harmful effects have not been observed in breastfed infants exposed to CHCs through breast milk. CHCs can reduce milk production in breastfeeding mothers. This is less likely to occur once breastfeeding is well-established; however, it can occur at any time in some women.
When possible, advise the nursing mother to use non-estrogen-containing contraception until she has completely weaned her child. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for NuvaRing and any potential adverse effects on the breastfed child from NuvaRing or from the underlying maternal condition.
8.4 Pediatric Use
Safety and efficacy of NuvaRing have been established in women of reproductive age. Efficacy is expected to be the same for postpubertal adolescents under the age of 18 and for users 18 years and older. Use of this product before menarche is not indicated.
8.5 Geriatric Use
NuvaRing has not been studied in postmenopausal women and is not indicated in this population.
8.6 Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of NuvaRing has not been studied. Steroid hormones may be poorly metabolized in patients with impaired liver function. Acute or chronic disturbances of liver function may necessitate the discontinuation of CHC use until markers of liver function return to normal. [See Contraindications (4) and Warnings and Precautions (5.3).]
10 OVERDOSAGE
There have been no reports of serious ill effects from overdose of CHCs. Overdosage may cause withdrawal bleeding in females and nausea. If the ring breaks, it does not release a higher dose of hormones. In case of suspected overdose, all NuvaRing rings should be removed and symptomatic treatment given.
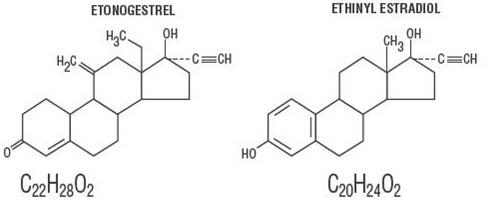
11 DESCRIPTION
NuvaRing (etonogestrel/ethinyl estradiol vaginal ring) is a non-biodegradable, flexible, transparent, colorless to almost colorless, combination contraceptive vaginal ring containing two active components, a progestin, etonogestrel (13-ethyl-17-hydroxy-11-methylene-18,19-dinor-17α-pregn-4-en-20-yn-3-one) and an estrogen, ethinyl estradiol (19-nor-17α-pregna-1,3,5(10)-trien-20-yne-3,17-diol). When placed in the vagina, each ring releases on average 0.120 mg/day of etonogestrel and 0.015 mg/day of ethinyl estradiol over a three-week period of use. NuvaRing is made of ethylene vinylacetate copolymers (28% and 9% vinylacetate) and magnesium stearate and contains 11.7 mg etonogestrel and 2.7 mg ethinyl estradiol. NuvaRing is not made with natural rubber latex. NuvaRing has an outer diameter of 54 mm and a cross-sectional diameter of 4 mm. The molecular weights for etonogestrel and ethinyl estradiol are 324.46 and 296.40, respectively.
The structural formulas are as follows:
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Combination hormonal contraceptives act by suppression of gonadotropins. Although the primary effect of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which reduce the likelihood of implantation).
12.3 Pharmacokinetics
Absorption
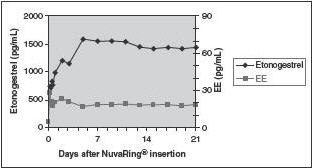
Etonogestrel: Etonogestrel released by NuvaRing is rapidly absorbed. The bioavailability of etonogestrel after vaginal administration is approximately 100%. The serum etonogestrel and ethinyl estradiol concentrations observed during three weeks of NuvaRing use are summarized in Table 2.
Ethinyl estradiol: Ethinyl estradiol released by NuvaRing is rapidly absorbed. The bioavailability of ethinyl estradiol after vaginal administration is approximately 56%, which is comparable to that with oral administration of ethinyl estradiol. The serum ethinyl estradiol concentrations observed during three weeks of NuvaRing use are summarized in Table 2.
| 1 week | 2 weeks | 3 weeks | |
|---|---|---|---|
| etonogestrel (pg/mL) | 1578 (408) | 1476 (362) | 1374 (328) |
| ethinyl estradiol (pg/mL) | 19.1 (4.5) | 18.3 (4.3) | 17.6 (4.3) |
The pharmacokinetic profile of etonogestrel and ethinyl estradiol during use of NuvaRing is shown in Figure 2.
Figure 2: Mean Serum Concentration-Time Profile of Etonogestrel and Ethinyl Estradiol during Three Weeks of NuvaRing Use
The pharmacokinetic parameters of etonogestrel and ethinyl estradiol were determined during one cycle of NuvaRing use in 16 healthy female subjects and are summarized in Table 3.
| Hormone | Cmax
pg/mL | Tmax
hr | t1/2
hr | CL L/hr |
|---|---|---|---|---|
| Cmax- maximum serum drug concentration Tmax- time at which maximum serum drug concentration occurs t1/2 - elimination half-life, calculated by 0.693/Kelim CL - apparent clearance |
||||
| etonogestrel | 1716 (445) | 200.3 (69.6) | 29.3 (6.1) | 3.4 (0.8) |
| ethinyl estradiol | 34.7 (17.5) | 59.3 (67.5) | 44.7 (28.8) | 34.8 (11.6) |
Prolonged use of NuvaRing: The mean serum etonogestrel concentration at the end of the fourth week of continuous use of NuvaRing was 1272 ± 311 pg/mL compared to a mean concentration range of 1578 ± 408 to 1374 ± 328 pg/mL at the end of weeks one to three. The mean serum ethinyl estradiol concentration at the end of the fourth week of continuous use of NuvaRing was 16.8 ± 4.6 pg/mL compared to a mean concentration range of 19.1 ± 4.5 to 17.6 ± 4.3 pg/mL at the end of weeks one to three.
Distribution
Metabolism
In vitro data shows that both etonogestrel and ethinyl estradiol are metabolized in liver microsomes by the cytochrome P450 3A4 isoenzyme. Ethinyl estradiol is primarily metabolized by aromatic hydroxylation, but a wide variety of hydroxylated and methylated metabolites are formed. These are present as free metabolites and as sulfate and glucuronide conjugates. The hydroxylated ethinyl estradiol metabolites have weak estrogenic activity. The biological activity of etonogestrel metabolites is unknown.
Drug Interactions
[See also Drug Interactions (7).]
The drug interactions of NuvaRing were evaluated in several studies.
A single-dose vaginal administration of an oil-based 1200-mg miconazole nitrate capsule increased the serum concentrations of etonogestrel and ethinyl estradiol by approximately 17% and 16%, respectively. Following multiple doses of 200 mg miconazole nitrate by vaginal suppository or vaginal cream, the mean serum concentrations of etonogestrel and ethinyl estradiol increased by up to 40%.
A single-dose vaginal administration of 100-mg water-based nonoxynol-9 spermicide gel did not affect the serum concentrations of etonogestrel or ethinyl estradiol.
The serum concentrations of etonogestrel and ethinyl estradiol were not affected by concomitant administration of oral amoxicillin or doxycycline in standard dosages during 10 days of antibiotic treatment.
Tampon Use
The use of tampons had no effect on serum concentrations of etonogestrel and ethinyl estradiol during use of NuvaRing [see Dosage and Administration (2.5)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 24-month carcinogenicity study in rats with subdermal implants releasing 10 and 20 mcg etonogestrel per day, (approximately 0.3 and 0.6 times the systemic steady-state exposure of women using NuvaRing), no drug-related carcinogenic potential was observed.
Mutagenesis
Etonogestrel was not genotoxic in the in vitro Ames/Salmonella reverse mutation assay, the chromosomal aberration assay in Chinese hamster ovary cells or in the in vivo mouse micronucleus test.
Impairment of Fertility
A fertility study was conducted with etonogestrel in rats at approximately 600 times the anticipated daily vaginal human dose (~0.002 mg/kg/day). Treatment did not have any adverse effect on resulting litter parameters after cessation of treatment supporting the return to fertility after suppression with etonogestrel.
14 CLINICAL STUDIES
In three large one-year clinical trials enrolling 2,834 women aged 18-40 years, in North America, Europe, Brazil, and Chile, the racial distribution was 93% Caucasian, 5.0% Black, 0.8% Asian, and 1.2% Other. Women with BMI ≥ 30 kg/m2 were excluded from these studies.
Based on pooled data from the three trials, 2,356 women aged < 35 years completed 23,515 evaluable cycles of NuvaRing use (cycles in which no back-up contraception was used). The pooled pregnancy rate (Pearl Index) was 1.28 (95% CI [0.8, 1.9]) per 100 women-years of NuvaRing use. In the US study, the Pearl Index was 2.02 (95% CI [1.1, 3.4]) per 100 women-years of NuvaRing use.
Study data indicate the return of ovulation and spontaneous menstrual cycles in most women within a month after discontinuation of NuvaRing use.
15 REFERENCES
- Dinger, J et. al., Cardiovascular risk associated with the use of an etonogestrel-containing vaginal ring. Obstetrics & Gynecology 2013; 122(4): 800-808.
- Sidney, S. et. al., Recent combined hormonal contraceptives (CHCs) and the risk of thromboembolism and other cardiovascular events in new users. Contraception 2013; 87: 93–100.
- Combined hormonal contraceptives (CHCs) and the risk of cardiovascular endpoints. Sidney, S. (primary author) http://www.fda.gov/downloads/Drugs/DrugSafety/UCM277384.pdf, accessed 23-Aug-2013.
16 HOW SUPPLIED/STORAGE AND HANDLING
Each NuvaRing (etonogestrel/ethinyl estradiol vaginal ring) is individually packaged in a reclosable aluminum laminate sachet consisting of three layers, from outside to inside: polyester, aluminum foil, and low-density polyethylene. The ring should be replaced in this reclosable sachet after use and discarded in a waste receptacle out of the reach of children and pets. It should not be flushed down the toilet.
| Box of 3 sachets | NDC 0052-0273-03 |
| Box of 3 sachets | NDC 0052-0273-05 |
16.1 Storage
Prior to dispensing to the user, store refrigerated 2-8°C (36-46°F). After dispensing to the user, NuvaRing can be stored for up to 4 months at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature].
Avoid storing NuvaRing in direct sunlight or at temperatures above 30°C (86°F).
For the Dispenser: When NuvaRing is dispensed to the user, place an expiration date on the label. The date should not exceed either 4 months from the date of dispensing or the expiration date, whichever comes first.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Counsel patients regarding the following:
Increased risk of cardiovascular events
- Advise patients that cigarette smoking increases the risk of serious cardiovascular events from use of NuvaRing, and women who are over 35 years old and smoke should not use NuvaRing [see Boxed Warning].
- Inform patients that the increased risk of VTE compared to non-users of CHCs is greatest after initially starting a CHC or restarting (following a 4-week or greater CHC-free interval) the same or a different CHC [see Warnings and Precautions (5.1)].
Use and administration
- Inform patients that NuvaRing does not protect against HIV infection (AIDS) and other sexually transmitted infections.
- Advise patients on the proper usage of NuvaRing and what to do if she does not comply with the labeled timing of insertion and removal [see Dosage and Administration (2)].
- Advise patients to regularly check for the presence of NuvaRing in the vagina (for example, before and after intercourse) [see Dosage and Administration (2.3)].
Pregnancy
- Inform patients that NuvaRing is not to be used during pregnancy. If pregnancy is planned or occurs during treatment with NuvaRing, instruct the patient to discontinue NuvaRing use [see Use in Specific Populations (8.1)].
Use of additional contraception
- Inform patients that they need to use a barrier method of contraception when the ring is out for more than three continuous hours until NuvaRing has been used continuously for at least seven days [see Dosage and Administration (2.3)].
- Advise patients to use a back-up or alternative method of contraception when enzyme inducers are used with NuvaRing [see Drug Interactions (7.1)].
- Inform patients who start NuvaRing postpartum and have not yet had a normal period that they should use an additional non-hormonal method of contraception for the first seven days [see Dosage and Administration (2.2)].
Lactation
- Inform patients that CHCs may reduce breast milk production. This is less likely to occur if breastfeeding is well established [see Use in Specific Populations (8.2)].
Amenorrhea
- Inform patients that amenorrhea may occur. Rule out pregnancy in the event of amenorrhea if NuvaRing has been out of the vagina for more than three consecutive hours, if the ring-free interval was extended beyond one week, if the woman has missed a period for two or more consecutive cycles, and if the ring has been retained for longer than four weeks [see Warnings and Precautions (5.11)].
Disposal
- Advise patients on the proper disposal of a used NuvaRing [see How Supplied/Storage and Handling (16)].
Manufactured for: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
Manufactured by: N.V. Organon, Oss, The Netherlands, a subsidiary of
Merck & Co., Inc., Whitehouse Station, NJ 08889, USA
For patent information: www.merck.com/product/patent/home.html
Copyright © 2001-2020 Merck Sharp & Dohme B.V., a subsidiary of Merck & Co., Inc.
All rights reserved.
uspi-mk8342a-rng-2001r014
Patient Information
NuvaRing® (NEW-vah-ring)
(etonogestrel/ethinyl estradiol vaginal ring)
|
What is the most important information I should know about NuvaRing? Do not use NuvaRing if you smoke cigarettes and are over 35 years old. Smoking increases your risk of serious cardiovascular side effects (heart and blood vessel problems) from combination hormonal contraceptives (CHCs), including death from heart attack, blood clots or stroke. This risk increases with age and the number of cigarettes you smoke. |
Hormonal birth control methods help to lower the chances of becoming pregnant. They do not protect against HIV infection (AIDS) and other sexually transmitted infections.
What is NuvaRing?
NuvaRing (NEW-vah-ring) is a flexible birth control vaginal ring used to prevent pregnancy.
NuvaRing contains a combination of a progestin and estrogen, 2 kinds of female hormones. Birth control methods that contain both an estrogen and a progestin are called combination hormonal contraceptives (CHCs).
How well does NuvaRing work?
Your chance of getting pregnant depends on how well you follow the directions for using NuvaRing. The better you follow the directions, the less chance you have of getting pregnant.
Based on the results of a US clinical study, approximately 1 to 3 women out of 100 women may get pregnant during the first year they use NuvaRing.
The following chart shows the chance of getting pregnant for women who use different methods of birth control. Each box on the chart contains a list of birth control methods that are similar in effectiveness. The most effective methods are at the top of the chart. The box on the bottom of the chart shows the chance of getting pregnant for women who do not use birth control and are trying to get pregnant.
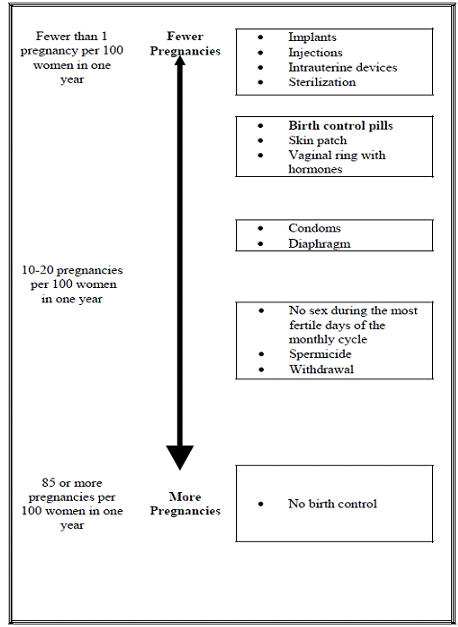
Who should not use NuvaRing?
Do not use NuvaRing if you:
- smoke and are over 35 years old
- have or have had blood clots in your arms, legs, eyes, or lungs
- have an inherited problem with your blood that makes it clot more than normal
- have had a stroke
- have had a heart attack
- have certain heart valve problems or heart rhythm problems that can cause blood clots to form in the heart
- have high blood pressure that medicine can't control
- have diabetes with kidney, eye, nerve, or blood vessel damage
- have certain kinds of severe migraine headaches with aura, numbness, weakness, or changes in vision, or have any migraine headaches if you are over age 35
- have liver disease, including liver tumors
- take any Hepatitis C drug combination containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir. This may increase levels of the liver enzyme "alanine aminotransferase" (ALT) in the blood
- have unexplained vaginal bleeding
- are pregnant or think you may be pregnant. NuvaRing is not for pregnant women.
- have or have had breast cancer or any cancer that is sensitive to female hormones
- are allergic to etonogestrel, ethinyl estradiol or any of the ingredients in NuvaRing. See the list of ingredients in NuvaRing at the end of this leaflet.
Hormonal birth control methods may not be a good choice for you if you have ever had jaundice (yellowing of the skin or eyes) caused by pregnancy or related to previous use of hormonal birth control.
Tell your healthcare provider if you have ever had any of the conditions listed above. Your healthcare provider can suggest another method of birth control.
What should I tell my healthcare provider before using NuvaRing?
Before you use NuvaRing tell your healthcare provider if you:
- have any medical conditions
- smoke
- are pregnant or think you are pregnant
- recently had a baby
- recently had a miscarriage or abortion
- have a family history of breast cancer
- have or have had breast nodules, fibrocystic disease, an abnormal breast x-ray, or abnormal mammogram
- use tampons and have a history of toxic shock syndrome
- have been diagnosed with depression
- have had liver problems including jaundice during pregnancy
- have or have had elevated cholesterol or triglycerides
- have or have had gallbladder, liver, heart, or kidney disease
- have diabetes
- have a history of jaundice (yellowing of the skin or eyes) caused by pregnancy (also called cholestasis of pregnancy)
- have a history of scanty or irregular menstrual periods
- have any condition that makes the vagina become irritated easily
- have or have had high blood pressure
- have or have had migraines or other headaches or seizures
- are scheduled for surgery. NuvaRing may increase your risk of blood clots after surgery. You should stop using NuvaRing at least 4 weeks before you have surgery and not restart it until at least 2 weeks after your surgery.
- are scheduled for any laboratory tests. Certain blood tests may be affected by hormonal birth control methods.
- are breastfeeding or plan to breastfeed. Hormonal birth control methods that contain estrogen, like NuvaRing, may decrease the amount of milk you make. A small amount of hormones from NuvaRing may pass into your breast milk. Consider another non-hormonal method of birth control until you are ready to stop breastfeeding.
- have (or have ever had) an allergic reaction while using NuvaRing, including hives, swelling of the face, lips, tongue, and/or throat causing difficulty in breathing or swallowing (anaphylaxis and/or angioedema).
Tell your healthcare provider about all medicines and herbal products you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
Some medicines and herbal products may make hormonal birth control less effective, including, but not limited to:
- certain anti-seizure medicines (such as barbiturates, carbamazepine, felbamate, oxcarbazepine, phenytoin, rufinamide and topiramate)
- medicine to treat fungal infections (griseofulvin)
- certain combinations of HIV medicines, (such as nelfinavir, ritonavir, darunavir/ritonavir, (fos)amprenavir/ritonavir, lopinavir/ritonavir, and tipranavir/ritonavir)
- certain hepatitis C (HCV) medicines (such as boceprevir and telaprevir)
- non-nucleoside reverse transcriptase inhibitors (such as efavirenz and nevirapine)
- medicine to treat tuberculosis (such as rifampicin and rifabutin)
- medicine to treat high blood pressure in the vessels of the lung (bosentan)
- medicine to treat chemotherapy-induced nausea and vomiting (aprepitant)
- St John's wort
Use an additional barrier contraceptive method (such as a male condom with spermicide) when you take medicines that may make NuvaRing less effective. Since the effect of another medicine on NuvaRing may last up to 28 days after stopping the medicine, it is necessary to use the additional barrier contraceptive method for that long to help prevent you from becoming pregnant. While using NuvaRing, you should not use certain female barrier contraceptive methods such as a vaginal diaphragm, cervical cap or female condom as your back-up method of birth control because NuvaRing may interfere with the correct placement and position of a diaphragm, cervical cap or female condom.
Some medicines and grapefruit juice may increase the level of ethinyl estradiol in your blood if used together, including:
- the pain reliever acetaminophen
- ascorbic acid (vitamin C)
- medicines that affect how your liver breaks down other medicines (such as itraconazole, ketoconazole, voriconazole, fluconazole, clarithromycin, erythromycin, and diltiazem)
- certain HIV medicines (atazanavir/ritonavir and indinavir)
- non-nucleoside reverse transcriptase inhibitors (such as etravirine)
- medicines to lower cholesterol such as atorvastatin and rosuvastatin
Hormonal birth control methods may interact with lamotrigine, a medicine used for seizures. This may increase the risk of seizures, so your healthcare provider may need to adjust your dose of lamotrigine.
Women on thyroid replacement therapy may need increased doses of thyroid hormone.
Ask your healthcare provider if you are not sure if you take any of the medicines listed above. Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I use NuvaRing?
- Read the Instructions for Use at the end of this Patient Information that comes with your NuvaRing for information about the right way to use NuvaRing.
- Use NuvaRing exactly as your healthcare provider tells you to use it.
- NuvaRing is used in a 4-week cycle.
- Insert 1 NuvaRing in the vagina and keep it in place for 3 weeks (21 days).
Regularly check that NuvaRing is in your vagina (for example, before and after intercourse) to ensure that you are protected from pregnancy.
- Remove the NuvaRing for a 1-week break (7 days). During the 1-week break (7 days), you will usually have your menstrual period.
Note: Insert and remove NuvaRing on the same day of the week and at the same time:
- For example, if you insert your NuvaRing on a Monday at 8:00 am, you should remove it on the Monday 3 weeks later at 8:00 am.
- After your 1-week (7 days) break, you should insert a new NuvaRing on the next Monday at 8:00 am.
- Insert 1 NuvaRing in the vagina and keep it in place for 3 weeks (21 days).
- While using NuvaRing, you should not use certain female barrier contraceptive methods such as a vaginal diaphragm, cervical cap or female condom as your back-up method of birth control because NuvaRing may interfere with the correct placement and position of a diaphragm, cervical cap or female condom.
- Ring breakage has occurred when also using a vaginal product such as a lubricant or treatment for infection (see "What should I do if my NuvaRing comes out of my vagina?"). Use of spermicides or vaginal yeast products will not make NuvaRing less effective at preventing pregnancy.
- Use of tampons will not make NuvaRing less effective or stop NuvaRing from working.
- If NuvaRing has been left inside your vagina for more than 4 weeks (28 days), you may not be protected from pregnancy and you should see your healthcare provider to be sure you are not pregnant. Until you know the results of your pregnancy test, you should use an extra method of birth control, such as male condoms with spermicide, until the new NuvaRing has been in place for 7 days in a row.
- Do not use more than 1 NuvaRing at a time. Too much hormonal birth control medicine in your body may cause nausea, vomiting, or vaginal bleeding.
Your healthcare provider should examine you at least 1 time a year to see if you have any signs of side effects from using NuvaRing.
What are the possible side effects of using NuvaRing?
See "What is the most important information I should know about NuvaRing?"
NuvaRing may cause serious side effects, including:
-
blood clots. Like pregnancy, combination hormonal birth control methods increase the risk of serious blood clots (see following graph), especially in women who have other risk factors, such as smoking, obesity, or age greater than 35. This increased risk is highest when you first start using a combination hormonal birth control method or when you restart the same or different combination hormonal birth control method after not using it for a month or more. Talk with your healthcare provider about your risk of getting a blood clot before using NuvaRing or before deciding which type of birth control is right for you.
- In some studies of women who used NuvaRing, the risk of getting a blood clot was similar to the risk in women who used combination birth control pills.
- Other studies have reported that the risk of blood clots was higher for women who use combination birth control pills containing desogestrel (a progestin similar to the progestin in NuvaRing) than for women who use combination birth control pills that do not contain desogestrel.
-
It is possible to die or be permanently disabled from a problem caused by a blood clot, such as heart attack or stroke. Some examples of serious blood clots are blood clots in the:
- legs (deep vein thrombosis)
- lungs (pulmonary embolus)
- eyes (loss of eyesight)
- heart (heart attack)
- brain (stroke)
- To put the risk of developing a blood clot into perspective: If 10,000 women who are not pregnant and do not use hormonal birth control are followed for one year, between 1 and 5 of these women will develop a blood clot. The figure below shows the likelihood of developing a serious blood clot for women who are not pregnant and do not use hormonal birth control, for women who use hormonal birth control, for pregnant women, and for women in the first 12 weeks after delivering a baby.
-
Likelihood of Developing a Serious Blood Clot (Venous Thromboembolism [VTE])
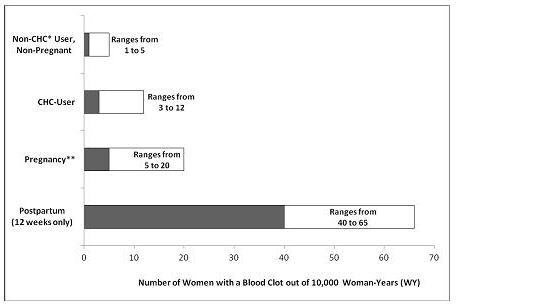
- *CHC=combination hormonal contraception
- **Pregnancy data based on actual duration of pregnancy in the reference studies. Based on a model assumption that pregnancy duration is nine months, the rate is 7 to 27 per 10,000 WY.
-
Call your healthcare provider right away if you have:
- leg pain that does not go away
- sudden shortness of breath
- sudden blindness, partial or complete
- severe pain or pressure in your chest
- sudden, severe headache unlike your usual headaches
- weakness or numbness in an arm or leg, or trouble speaking
- yellowing of the skin or eyeballs
Other serious risks include:
- Toxic Shock Syndrome (TSS). Some of the symptoms are much the same as the flu, but they can become serious very quickly. Call your healthcare provider or get emergency treatment right away if you have the following symptoms:
- sudden high fever
- vomiting
- diarrhea
- a sunburn-like rash
- muscle aches
- dizziness
- fainting or feeling faint when standing up
- allergic reaction, including hives, swelling of the face, lips, tongue, and/or throat causing difficulty in breathing or swallowing (anaphylaxis and/or angioedema)
- liver problems, including liver tumors
- high blood pressure
- gallbladder problems
- accidental insertion into bladder
- symptoms of a problem called angioedema if you already have a family history of angioedema
The most common side effects of NuvaRing are:
- tissue irritation inside your vagina or on your cervix
- headache (including migraine)
- mood changes (including depression, especially if you had depression in the past). Call your healthcare provider immediately if you have any thoughts of harming yourself.
- NuvaRing problems, including the ring slipping out or causing discomfort
- nausea and vomiting
- vaginal discharge
- weight gain
- vaginal discomfort
- breast pain, discomfort, or tenderness
- painful menstrual periods
- abdominal pain
- acne
- less sexual desire
Some women have spotting or light bleeding during NuvaRing use. If these symptoms occur, do not stop using NuvaRing. The problem will usually go away. If it doesn't go away, check with your healthcare provider.
Other side effects seen with NuvaRing include breast discharge; vaginal injury (including pain, discomfort, and bleeding) associated with broken rings; and penis discomfort of the partner (such as irritation, rash, itching).
Less common side effects seen with combination hormonal birth control include:
- Blotchy darkening of your skin, especially on your face
- High blood sugar, especially in women who already have diabetes
- High fat (cholesterol, triglycerides) levels in the blood
There have been reports of the ring becoming stuck to the vaginal tissue and having to be removed by a healthcare provider. Call your healthcare provider if you are unable to remove your NuvaRing.
Tell your healthcare provider about any side effect that bothers you or that does not go away. These are not all the possible side effects of NuvaRing. For more information, ask your healthcare provider or pharmacist. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store NuvaRing and throw away used NuvaRings?
- Store NuvaRing at room temperature between 68°F to 77°F (20°C to 25°C).
- Store NuvaRing at room temperature for up to 4 months after you receive it. Throw NuvaRing away if the expiration date on the label has passed.
- Do not store NuvaRing above 86°F (30°C).
- Avoid direct sunlight.
- Place the used NuvaRing in the re-closable foil pouch and properly throw it away in your household trash out of the reach of children and pets. Do not flush your used NuvaRing down the toilet.
Keep NuvaRing and all medicines out of the reach of children.
General information about the safe and effective use of NuvaRing
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information. Do not use NuvaRing for a condition for which it was not prescribed. Do not give NuvaRing to other people. It may harm them.
This leaflet summarizes the most important information about NuvaRing. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about NuvaRing that is written for health professionals.
For more information on NuvaRing and the applicator for NuvaRing, go to www.nuvaring.com or call 1-800-622-4477.
What are the ingredients in NuvaRing?
Active ingredients: etonogestrel and ethinyl estradiol
Inactive ingredients: ethylene vinylacetate copolymers (28% and 9% vinylacetate) and magnesium stearate.
NuvaRing is not made with natural rubber latex.
Do Hormonal Birth Control Methods Cause Cancer?
Hormonal birth control methods do not seem to cause breast cancer. However, if you have breast cancer now or have had it in the past, do not use hormonal birth control, including NuvaRing, because some breast cancers are sensitive to hormones.
Women who use hormonal birth control methods may have a slightly higher chance of getting cervical cancer. However, this may be due to other reasons such as having more sexual partners.
What should I know about my period when using NuvaRing?
When you use NuvaRing you may have bleeding and spotting between periods, called unplanned bleeding. Unplanned bleeding may vary from slight staining between menstrual periods to breakthrough bleeding, which is a flow much like a regular period. Unplanned bleeding occurs most often during the first few months of NuvaRing use, but may also occur after you have been using NuvaRing for some time. Such bleeding may be temporary and usually does not indicate any serious problems. It is important to continue using the ring on schedule. If the unplanned bleeding or spotting is heavy or lasts for more than a few days, you should discuss this with your healthcare provider.
What if I miss my regular scheduled period when using NuvaRing?
Some women miss periods on hormonal birth control, even when they are not pregnant. Consider the possibility that you may be pregnant if:
- you miss a period and NuvaRing was out of the vagina for more than 3 hours during the 3 weeks (21 days) of ring use
- you miss a period and waited longer than 1 week to insert a new ring
- you have followed the instructions and you miss 2 periods in a row
- you have left NuvaRing in place for longer than 4 weeks (28 days)
What if I want to become pregnant?
You may stop using NuvaRing whenever you wish. Consider a visit with your healthcare provider for a pre-pregnancy checkup before you stop using NuvaRing.
Instructions for Use
NuvaRing (NEW-vah-ring)
(etonogestrel/ethinyl estradiol vaginal ring)
Read these Instructions for Use before you start using NuvaRing and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your treatment.
How should I start using NuvaRing?
If you are not currently using hormonal birth control, you have 2 ways to start using NuvaRing. Choose the best way for you:
- First Day Start: Insert NuvaRing on the first day of your menstrual period. You will not need to use another birth control method since you are using NuvaRing on the first day of your menstrual period.
- Day 2 to Day 5 Cycle Start: You may choose to start NuvaRing on days 2 to 5 of your menstrual period. Make sure you also use an extra method of birth control (barrier method), such as male condoms with spermicide for the first 7 days of NuvaRing use in the first cycle.
If you are changing from a birth control pill or patch to NuvaRing:
If you have been using your birth control method correctly and are certain that you are not pregnant, you can change to NuvaRing any day. Do not start NuvaRing any later than the day you would start your next birth control pill or apply your patch.
If you are changing from a progestin-only birth control method, such as a minipill, implant or injection or from an intrauterine system (IUS):
- You may switch from a minipill on any day. Start using NuvaRing on the day that you would have taken your next minipill.
- You should switch from an implant or the IUS and start using NuvaRing on the day that you remove the implant or IUS.
- You should switch from an injectable and start using NuvaRing on the day when your next injection would be due.
If you are changing from a minipill, implant or injection or from an intrauterine system (IUS), you should use an extra method of birth control, such as a male condom with spermicide during the first 7 days of using NuvaRing.
If you start using NuvaRing after an abortion or miscarriage:
- Following a first trimester abortion or miscarriage: You may start NuvaRing within 5 days following a first trimester abortion or miscarriage (the first 12 weeks of pregnancy). You do not need to use an additional birth control method.
- If you do not start NuvaRing within 5 days after a first trimester abortion or miscarriage, use a non-hormonal birth control method, such as male condoms and spermicide, while you wait for your period to start. Begin NuvaRing at the time of your next menstrual period. Count the first day of your menstrual period as "Day 1" and start NuvaRing one of the following 2 ways below.
- First Day Start: Insert NuvaRing on the first day of your menstrual period. You will not need to use another birth control method since you are using NuvaRing on the first day of your menstrual period.
- Day 2 to Day 5 Cycle Start: You may choose to start NuvaRing on Days 2 to 5 of your menstrual period. Make sure you also use an extra method of birth control (barrier method), such as male condoms with spermicide for the first 7 days of NuvaRing use in the first cycle.
- Following a second trimester abortion or miscarriage: You may start using NuvaRing no sooner than 4 weeks (28 days) after a second trimester abortion (after the first 12 weeks of pregnancy).
If you are starting NuvaRing after childbirth:
- You may start using NuvaRing no sooner than 4 weeks (28 days) after having a baby if you are not breastfeeding.
- If you have not gotten your menstrual period after childbirth, you should talk to your healthcare provider. You may need a pregnancy test to make sure you are not pregnant before you start using NuvaRing.
- Use another birth control method such as male condoms with spermicide for the first 7 days in addition to NuvaRing.
If you are breastfeeding you should not use NuvaRing. Use other birth control methods until you are no longer breastfeeding.
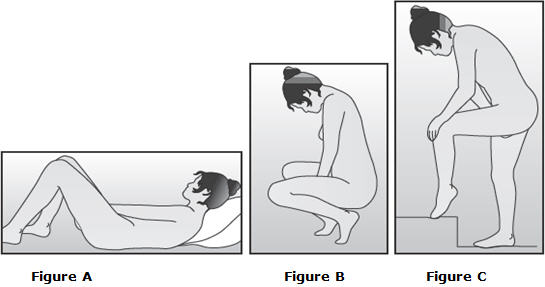
Step 1. Choose a position for insertion of NuvaRing.
- Choose the position that is comfortable for you. For example, lying down, squatting, or standing with 1 leg up (See Figures A, B, and C).
| Positions for NuvaRing insertion |
 |
Step 2. Open the pouch to remove your NuvaRing.
- Each NuvaRing comes in a re-sealable foil pouch.
- Wash and dry your hands before removing NuvaRing from the foil pouch.
- Open the foil pouch at either notch near the top.
- Keep the foil pouch so you can place your used NuvaRing in it before you throw it away in your household trash.
Step 3. Prepare NuvaRing for insertion.
- Hold NuvaRing between your thumb and index finger and press the sides of the ring together (See Figures D and E).
Step 4. Insert NuvaRing into your vagina.
- Insert the folded NuvaRing into your vagina and gently push it further up into your vagina using your index finger (See Figures F and G). Alternatively, the applicator for NuvaRing (available separately) may be used to help you insert the ring [see Applicator for NuvaRing Instructions for Use].
- When you insert NuvaRing it may be in different positions in your vagina, but NuvaRing does not have to be in an exact position for it to work (See Figures H and I).
- NuvaRing may move around slightly within your vagina. This is normal. Although some women may be aware of NuvaRing in the vagina, most women do not feel it when it is in place.
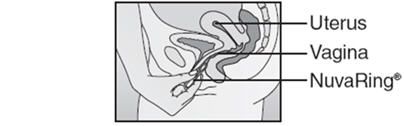 Figure F | ||
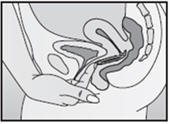 Figure G | 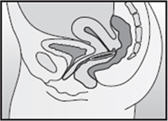 Figure H | 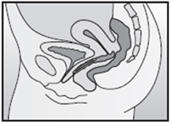 Figure I |
Inserting NuvaRing (Figure F, Figure G) and positioning NuvaRing (Figure H, Figure I)
Note:
- If the NuvaRing feels uncomfortable, you may not have pushed the ring into your vagina far enough. Use your finger to gently push the NuvaRing as far as you can into your vagina. There is no danger of NuvaRing being pushed too far up in the vagina or getting lost (See Figure G).
- Some women have accidentally inserted NuvaRing into their bladder. If you have pain during or after insertion and you cannot find NuvaRing in your vagina, call your healthcare provider right away.
- Regularly check that NuvaRing is in your vagina (for example, before and after intercourse) to ensure you are protected from pregnancy.
Step 5. How do I remove NuvaRing?
- Wash and dry your hands.
- Choose the position that is most comfortable for you (See Figures A, B, and C).
- Put your index finger into your vagina and hook it through the NuvaRing. Gently pull downward and forward to remove the NuvaRing and pull it out (See Figure J).
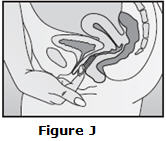 |
Step 6. Throw away the used NuvaRing.
- Place the used NuvaRing in the re-sealable foil pouch and put it in a trash can out of the reach of children and pets.
- Do not throw NuvaRing in the toilet.
What else should I know about using NuvaRing?
What if I leave NuvaRing in too long?
- If you leave NuvaRing in your vagina for up to 4 weeks (28 days) you will still be getting pregnancy protection. Remove your old NuvaRing for 1 week (7 days) and insert a new NuvaRing 1 week (7 days) later (See Steps 1 through 4).
- If you leave NuvaRing in your vagina longer than 4 weeks (28 days), remove the ring and check to make sure you are not pregnant.
If you are not pregnant, insert a new NuvaRing (See Steps 1 through 4). You must use another birth control method, such as male condoms with spermicide, until the new NuvaRing has been used for 7 days in a row.
What should I do if my NuvaRing comes out of my vagina?
NuvaRing can slip or accidentally come out (expelled) of your vagina, for example, during sexual intercourse, bowel movements, use of tampons, or if it breaks.
- NuvaRing may break causing the ring to lose its shape. If the ring stays in your vagina this should not lower NuvaRing's effectiveness at preventing pregnancy.
- If NuvaRing breaks and slips out of your vagina, throw the broken ring in your household trash out of the reach of children and pets.
- Insert a new NuvaRing (See Steps 1 through 4).
- You should pay attention when removing a tampon to be sure that your NuvaRing is not accidentally pulled out.
- Be sure to insert NuvaRing before inserting a tampon.
- If you accidentally pull out your NuvaRing while using tampons, rinse your NuvaRing in cool to lukewarm (not hot) water and insert it again right away.
- NuvaRing can be pushed out of (expelled from) your vagina, for example, during sexual intercourse or during a bowel movement.
- If the expelled ring has been out of your vagina for less than 3 hours, rinse the expelled NuvaRing in cool to lukewarm (not hot) water and insert it again right away.
- If the expelled NuvaRing has been out of your vagina for more than 3 continuous hours:
- During Weeks 1 and 2, you may not be protected from pregnancy. Reinsert the ring as soon as you remember (See Steps 1 through 4). Use another birth control method, such as male condoms with spermicide, until the ring has been in place for 7 days in a row.
-
During Week 3, do not reinsert the NuvaRing that has been out of your vagina; but throw it away in your household trash away from children and pets. Use another birth control method, such as male condoms with spermicide, until the new NuvaRing has been used for 7 days in a row, following one of the two options below:
- -
- Option 1. Insert a new ring right away to start your next 21 Day NuvaRing use cycle. You may not have your regular period, but you may have spotting or vaginal bleeding.
- -
- Option 2. Insert a new ring no later than 7 days from the time the previous ring was removed or expelled. During this time, you may have your period.
Note: You should only choose to do option 2 if you used NuvaRing for 7 days in a row, prior to the day that your previous NuvaRing was accidentally removed or expelled.
- If NuvaRing was out of the vagina for an unknown amount of time, you may not be protected from pregnancy. Perform a pregnancy test prior to inserting a new ring and consult your healthcare provider.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Manufactured for: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
Manufactured by: N.V. Organon, Oss, The Netherlands, a subsidiary of
Merck & Co., Inc., Whitehouse Station, NJ 08889, USA
For patent information: www.merck.com/product/patent/home.html
Copyright © 2001-2019 Merck Sharp & Dohme B.V., a subsidiary of Merck & Co., Inc.
All rights reserved.
Revised: 05/2019
usppi-mk8342a-rng-1905r013
Instructions for Use
Applicator for NuvaRing® (NEW-vah-ring)
For optional use to help insert NuvaRing.
NuvaRing is not included with the applicator.
Important:
- The applicator is designed for use with NuvaRing.
- Do not reuse the applicator; it is designed for one time use.
- Do not share the applicator with others.
- If you accidently drop the applicator, wash it with cool to lukewarm water. Do not use hot water to wash the applicator.
- Throw the applicator away in the trash immediately after use.
- Do not flush the applicator down the toilet.
The applicator is not made with natural rubber latex.
Store the applicators between 41°F to 86°F (5°C to 30°C).
- 1:
-
Prepare
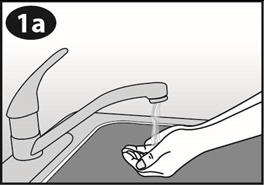
Wash your hands before opening the package. Open the package only when you are ready to use the applicator. Do not use if the contents or packaging are visibly damaged. 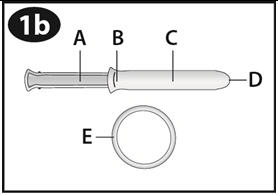
Look at the picture of the applicator to familiarize yourself with the parts of the applicator. - Plunger
- Finger Grip
- Barrel
- Barrel Opening
- NuvaRing (Not Included)
- 2:
-
Load & Position
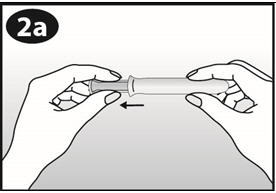
Pull the plunger back gently until it stops. 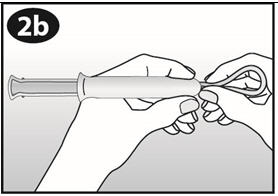
Squeeze opposite sides of the ring together and insert the ring into the barrel opening. 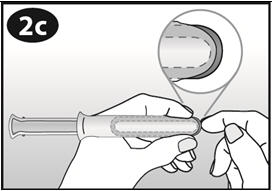
Gently push the ring into the barrel. The tip of the ring should stick out slightly from the barrel opening. 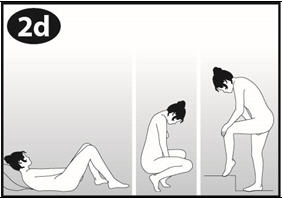
Choose the position for inserting the ring that is most comfortable for you, like lying down, squatting, or standing with one leg up. - 3:
-
Insert & Dispose
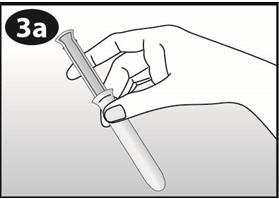
Place your thumb and middle finger on the finger grip. 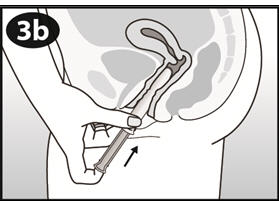
Gently insert the barrel into the vagina until your fingers (on the finger grip) touch your body. Then use your index finger to gently push the plunger all the way into the barrel. Some women have experienced a brief mild pinching feeling when using the applicator.
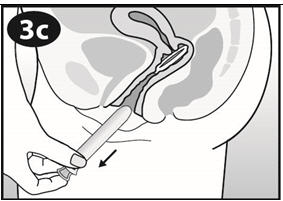
The ring is pushed out of the applicator. Gently remove the applicator. Please also read the Patient Information and Instructions for Use for NuvaRing. 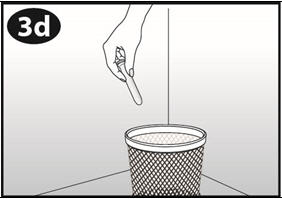
Make sure that the ring is not in the applicator. Dispose of (throw away) the used applicator in the trash can. Do not flush the applicator down the toilet. Do not reuse the applicator.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
Manufactured by: Sonoco Plastics B.V., Berkel en Rodenrijs, The Netherlands
For patent information: www.merck.com/product/patent/home.html
Copyright © 2016 Merck Sharp & Dohme B.V., a subsidiary of Merck & Co., Inc.
All rights reserved.
Revised: 09/2016
usifu-mkd920-1609r000
PRINCIPAL DISPLAY PANEL - 3 Ring Pouch Carton
For the Dispenser: Store refrigerated 2-8°C (36- 46°F).
NDC 0052-0273-05
NUVARING®
(etonogestrel/ethinyl estradiol vaginal ring)
delivers 0.120 mg/0.015 mg per day
For Vaginal Use
Keep out of the reach of children.
This product is intended to prevent
pregnancy. It does not protect against
HIV infection (AIDS) and other sexually
transmitted diseases.
Rx only
Contains 3 Rings

| NUVARING
etonogestrel and ethinyl estradiol insert, extended release |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Organon USA Inc. (078796541) |