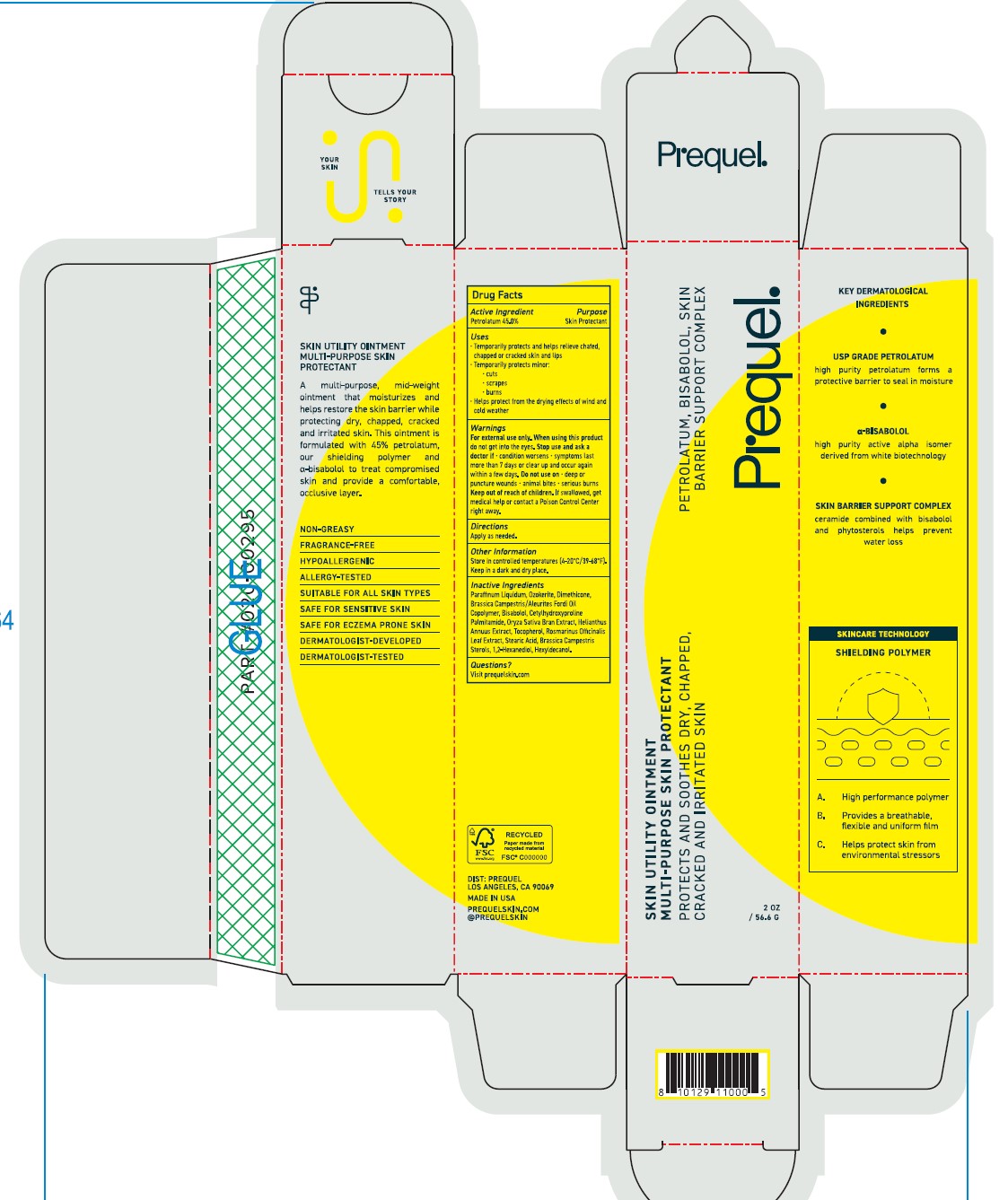
Uses
•Temporarily protects and helps releive chafed, chapped or craked skin and lips
•Temporarily protects minor:
•cuts
•scrapes
•burns
•Helps protect form the drying effects of wind and cold weather
Warnings
Fox external use only.
When using this product do not get into eyes
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days. Do not use on • deep or puncture woulds • animal bites • serious burns.
Keep out of reach of children. If swalled, get medical help or contact a Poison Control Center right away.
Keep out of reach of children. If swalled, get medical help or contact a Poison Control Center right away.
Inactive Ingredients
Parrafinum Liquidum, Ozokerite, Dimethicone, Brassica Campestric/Aleuites Fordi Oil Copolymer, Bisabolol, Cetylhydroxyproline Palmitamide, Oryza Sativa Bran Extract, Helianthus Annuus Extract, Tocopherol, Rosmarinus Officinalis Leaf Extract, Stearic Acid, Brassica Campestris Sterols, 1,2-Hexanediol, Hexyldecanol