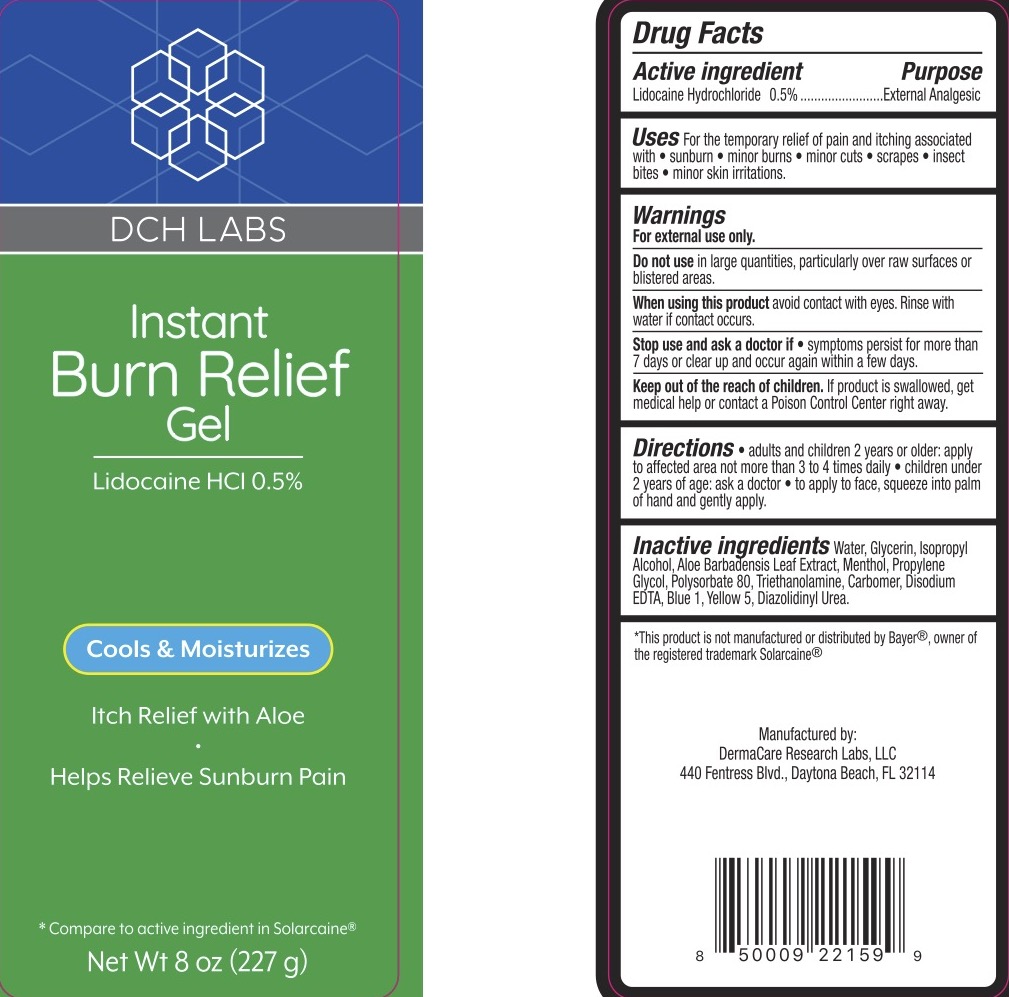
For the temporary relief of pain and itching due to sunburn, minor burns, insect bites, minor cuts, scrapes, and minor skin irritations.
For external use only.
Do not use in large quantities, particularly over raw surfaces or blistered areas.
When using this product avoid contact with eyes. Rinse with water if contact occurs.
Stop use and ask a doctor if the condition worsens or symptoms persist for more than 7 days or clear up and occur again within a few days.
Keep out of reach of children. If the product is swallowed, get medical help or contact a Poison Control Center right away.
Adults and children 2 years and older: apply to the affected area, not more than 3 to 4 times a day. Children under 2 years of age: consult a physician. To apply to face, squeeze into palm of hand and gently apply.