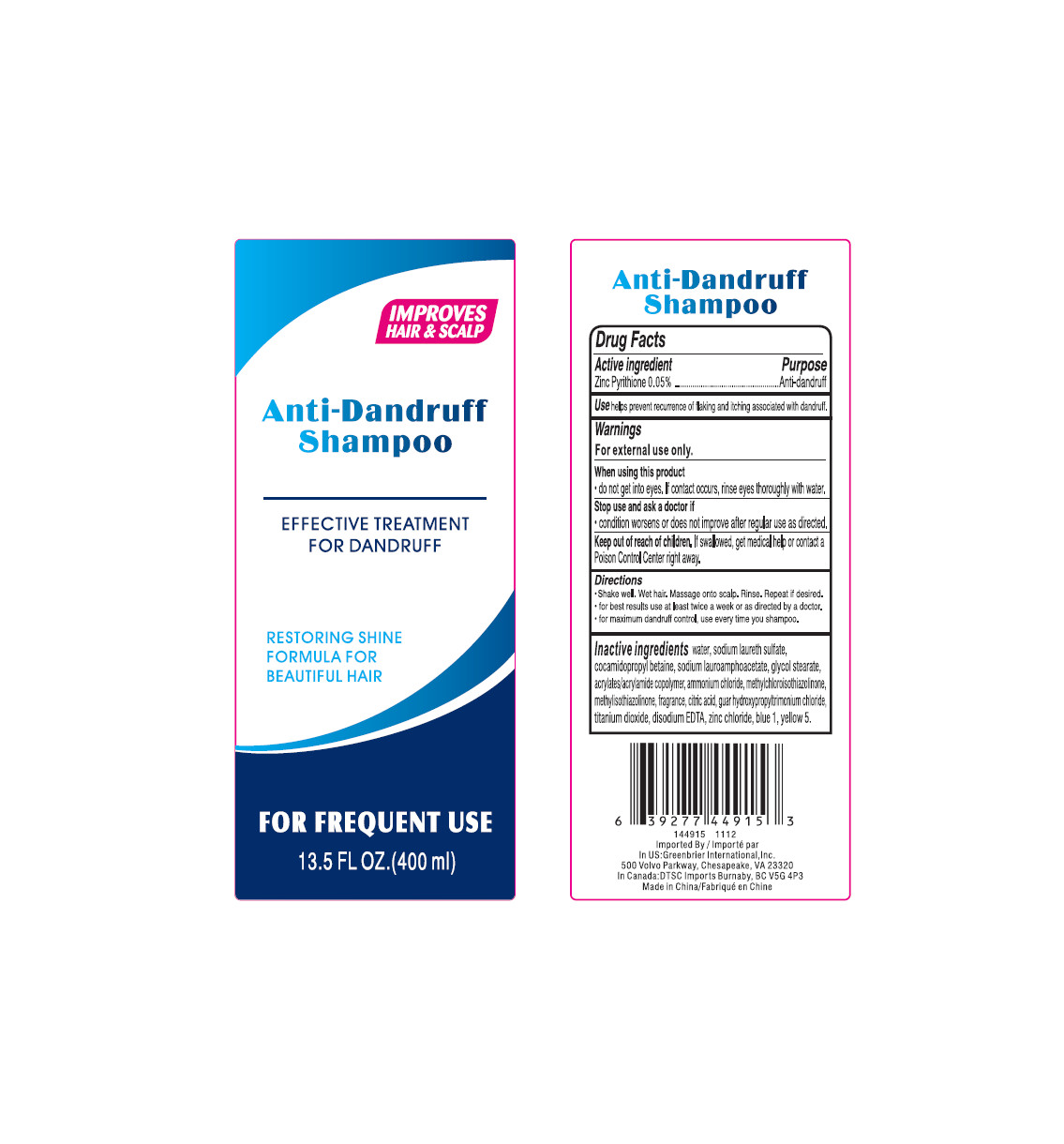
If swallowed, get medical help or contact a Poison Control Center right away.
Do not get into eyes, if contact occurs, rinse eyes thoroughly with water.
Shake well, wet hair, massage onto scalp. Rinse, repeat if desired.
For best results, use at least twice a week or as directed by a doctor.
For maximum dandruff control, use every time you shampoo.
For external use only.
Stop use and ask a doctor if Condition worsens or does not improve after regular use as directed.
water, sodium laureth sulfate, cocamidopropyl betaine, sodium lauroamphoacetate, glycol stearate, acrylates/acrylamide copolymer, ammonium chloride, methylchloroisothiazolinone, methylisothiazolinone, fragrance, citric acid, guar hydroxypropyltrimonium chloride, titanium dioxide, disodium EDTA, zinc chloride, blue 1, yellow 5