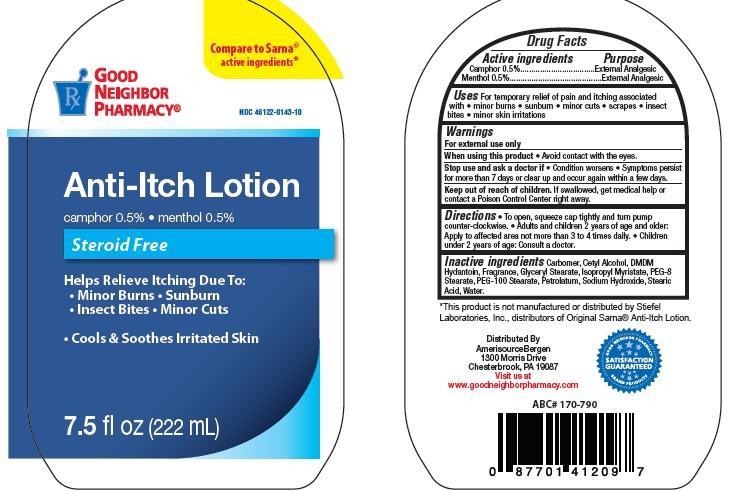
Uses
For temporary relief of pain and itching associated with • minor burns • sunburn • minor cuts • scra pes • insect
bites • minor skin irritations
Stop use and ask a doctor if
Condition worsens • Symptoms persist for more than 7 days or clear up and occur again within a few days.
Keep out of reach of children.
Keep out of reach of children.
If swallowed, get medical help or
contact a Poison Control Center right away.
Directions
To open, squeeze cap tightly and turn pump counter-clockwise. • Adults and children 2 years of age and older:
Apply to affected area not more than 3 to 4 times daily. • Children under 2 years of age: Consult a doctor.
Inactive ingredients
Carbomer, Cetyl Alcohol, DMDMHydantoin, Fragrance, Glyceryl Stearate, Isopropyl Myristate, PEG-8
Stearate, PEG-100 Stearate, Petrolatum, Sodium Hydroxide, Stearic Acid, Water.