PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 70771-1348-1 in bottle of 100 tablets
Methylprednisolone tablets, USP
Rx only
100 tablets

NDC 70771-1349-8 in bottle of 25 tablets
Methylprednisolone tablets, USP
Rx only
25 tablets

NDC 70771-1350-7 in bottle of 50 tablets
Methylprednisolone tablets, USP
Rx only
50 tablets

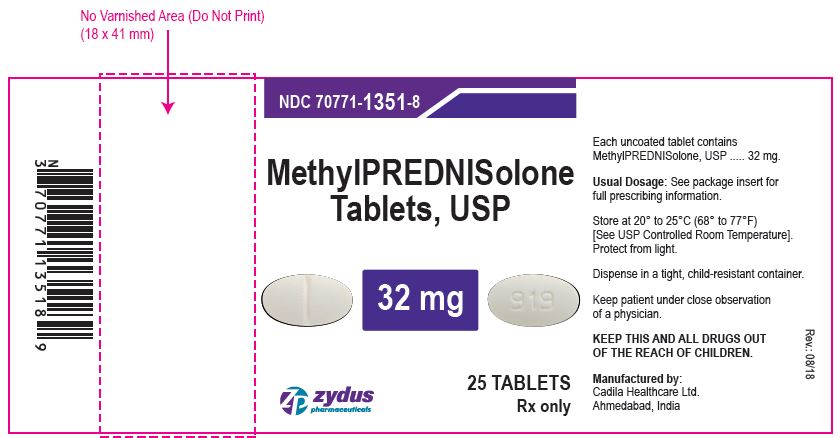
NDC 70771-1351-8 in bottle of 25 tablets
Methylprednisolone tablets, USP
Rx only
25 tablets