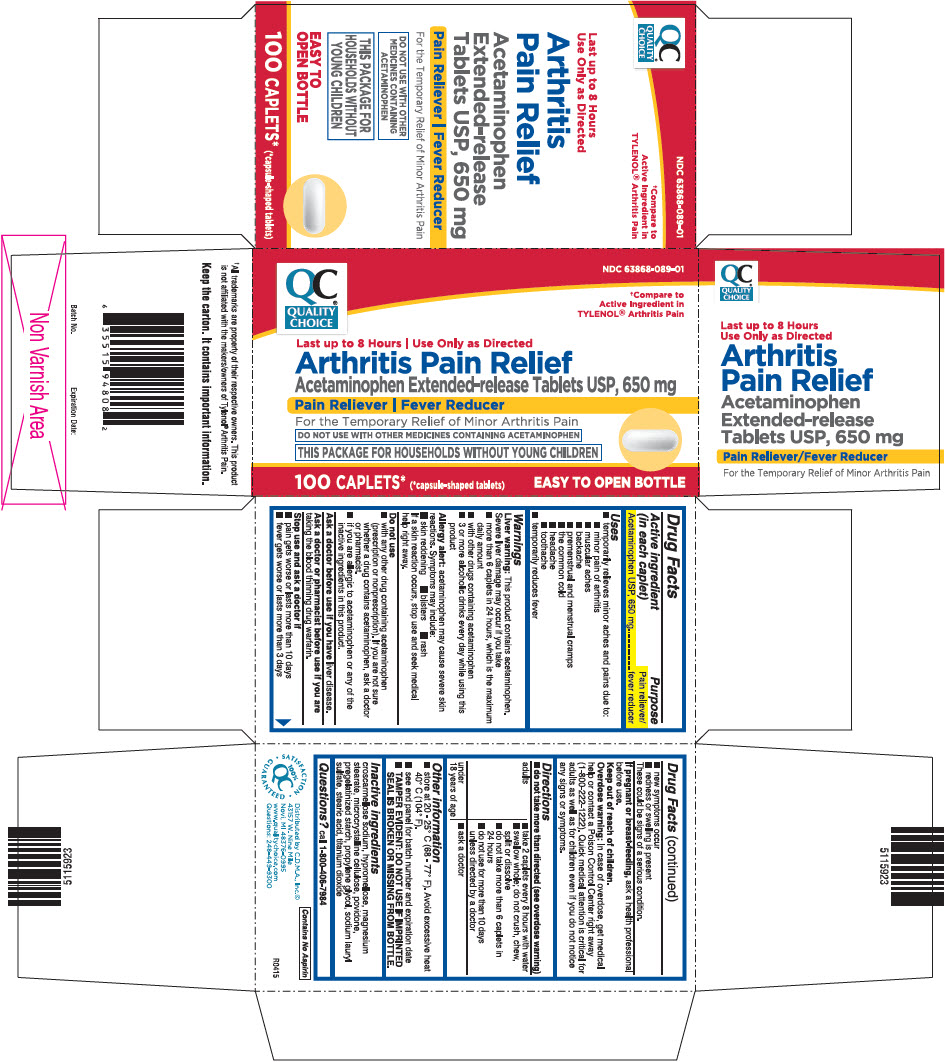
Uses
- temporarily relieves minor aches and pains due to:
- minor pain of arthritis
- muscular aches
- backache
- premenstrual and menstrual cramps
- the common cold
- headache
- toothache
- temporarily reduces fever
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 6 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product.
Directions
- do not take more than directed (see overdose warning)
| adults |
|
| under 18 years of age |
|
Other information
- store at 20 - 25° C (68 - 77° F). Avoid excessive heat 40° C (104° F).
- see end panel for batch number and expiration date
- TAMPER EVIDENT: DO NOT USE IF IMPRINTED SEAL IS BROKEN OR MISSING FROM BOTTLE.
Inactive ingredients
croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch, propylene glycol, sodium lauryl sulfate, stearic acid, titanium dioxide
PRINCIPAL DISPLAY PANEL - 100 Caplet Bottle Carton
QC®
QUALITY
CHOICE
NDC 63868-089-01
†Compare to
Active Ingredient in
TYLENOL® Arthritis Pain
Last up to 8 Hours | Use Only as Directed
Arthritis Pain Relief
Acetaminophen Extended-release Tablets USP, 650 mg
Pain Reliever | Fever Reducer
For the Temporary Relief of Minor Arthritis Pain
DO NOT USE WITH OTHER MEDICINES CONTAINING ACETAMINOPHEN
THIS PACKAGE FOR HOUSEHOLDS WITHOUT YOUNG CHILDREN
100 CAPLETS*
(*capsule-shaped tablets)
EASY TO OPEN BOTTLE