WARNINGS AND PRECAUTIONS SECTION
Should the material not be delivered to a NF customer, the material is “Not Approved for Human Drug Use.”
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Messer
Gases for Life
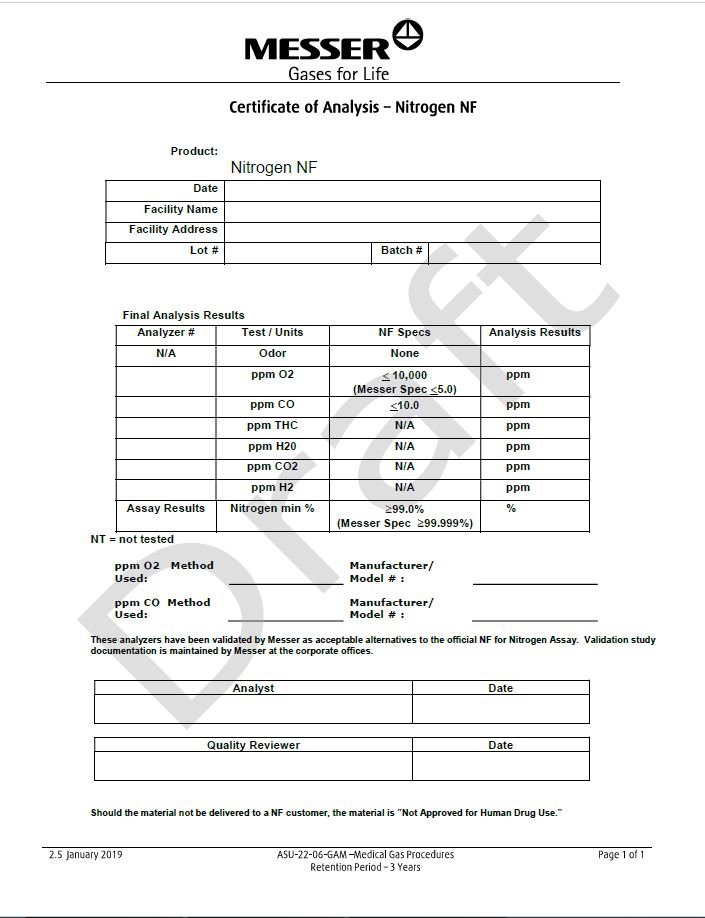
Certicate of Analysis - Nitrogen NF
Product: Nitrogen NF
|
Date |
|
||
|
Facility Name |
|
||
|
Facility Address |
|
||
|
Lot# |
|
Batch # |
|
Final Analysis Results
|
Analyzer # |
Test/Units |
NF Specs |
Analysis Results |
|
N/A |
Odor |
None |
|
|
|
ppm O2 |
< 10,000 (Messer Spec <5.0) |
ppm |
|
|
ppm CO |
< 10.0 |
ppm |
|
|
ppm THC |
N/A |
ppm |
|
|
ppm H2O |
N/A |
ppm |
|
|
ppm CO2 |
N/A |
ppm |
|
|
ppm H2 |
N/A |
ppm |
|
Assay Results |
Nitrogen min % |
> 99.0% (Messer Spec >99.999%) |
% |
NT= not tested
|
PPM O2 Method Used: __________________________ |
Manufacturer/Model #: __________________________ |
|
PPM O2 Method Used: __________________________ |
Manufacturer/Model #: __________________________ |
This analyzer has been validated by Messer as acceptable alternatives to the official NF for Nitrogen Assay. Validation study documentation is maintained by Messer at the corporate offices.
|
Analyst |
Date |
|
|
|
|
|
|
Quality Reviewer |
Date |
|
|
|
|
|
|
|
||
Should the material not be delivered to a NF customer, the material is “Not Approved for Human Drug Use.”
2.5 January 2019
ASU-22-06-GAM-Medical Gas Procedures
Page 1 of 1
Retention Period – 3 Years