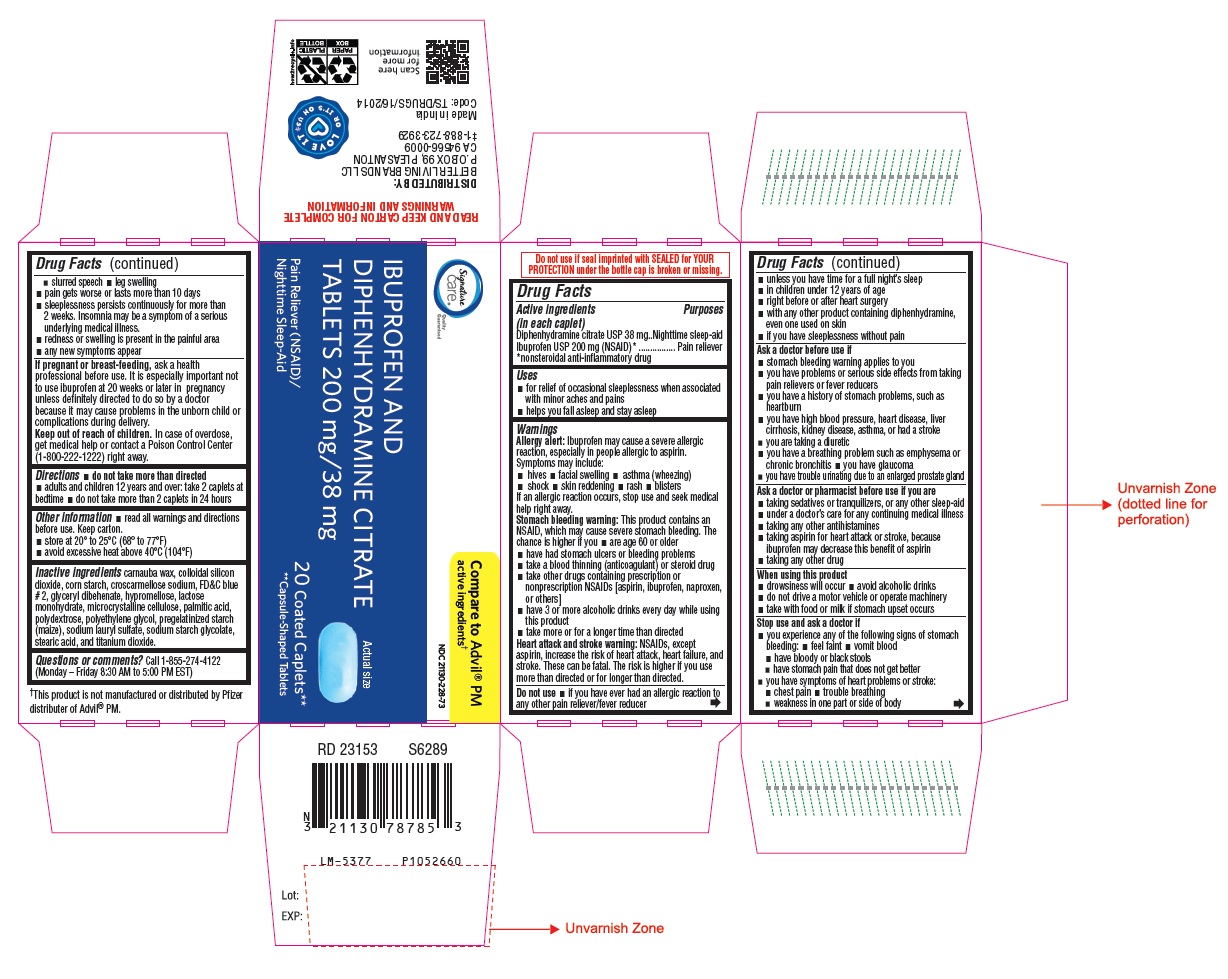
Drug Facts
Active ingredients
(in each caplet)
Diphenhydramine citrate USP 38 mg
Ibuprofen USP 200 mg (NSAID)*
*nonsteroidal anti-inflammatory drug
Uses
- for relief of occasional sleeplessness when associated with minor aches and pains
- helps you fall asleep and stay asleep
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Heart attack and stroke warning: NSAIDs, except aspirin, increase the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed.
Do not use
- if you have ever had an allergic reaction to any other pain reliever/fever reducer
- unless you have time for a full night's sleep
- in children under 12 years of age
- right before or after heart surgery
- with any other product containing diphenhydramine, even one used on skin
- if you have sleeplessness without pain
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have problems or serious side effects from taking pain relievers or fever reducers
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma, or had a stroke
- you are taking a diuretic
- you have a breathing problem such as emphysema or chronic bronchitis
- you have glaucoma
- you have trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers, or any other sleep-aid
- under a doctor's care for any continuing medical illness
- taking any other antihistamines
- taking aspirin for heart attack or stroke, because ibuprofen may decrease this benefit of aspirin
- taking any other drug
When using this product
- drowsiness will occur
- avoid alcoholic drinks
- do not drive a motor vehicle or operate machinery
- take with food or milk if stomach upset occurs
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- you have symptoms of heart problems or stroke:
- chest pain
- trouble breathing
- weakness in one part or side of body
- slurred speech
- leg swelling
- pain gets worse or lasts more than 10 days
- sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of a serious underlying medical illness.
- redness or swelling is present in the painful area
- any new symptoms appear
If pregnant or breast-feeding,
ask a health professional before use. It is especially important not to use ibuprofen at 20 weeks or later in pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
- do not take more than directed
- adults and children 12 years and over: take 2 caplets at bedtime
- do not take more than 2 caplets in 24 hours
Other information
- read all warnings and directions before use. Keep carton.
- store at 20° to 25°C (68° to 77°F)
- avoid excessive heat above 40°C (104°F)
Inactive ingredients
carnauba wax, colloidal silicon dioxide, corn starch, croscarmellose sodium, FD&C blue # 2, glyceryl dibehenate, hypromellose, lactose monohydrate, microcrystalline cellulose, palmitic acid, polydextrose, polyethylene glycol, pregelatinized starch (maize), sodium lauryl sulfate, sodium starch glycolate, stearic acid, and titanium dioxide.
Questions or comments?
Call 1-855-274-4122 (Monday – Friday 8:30 AM to 5:00 PM EST)
DISTRIBUTED BY:
BETTER LIVING BRANDS LLC
P.O.BOX 99, PLEASANTON
CA 94566-0009
‡1-888-723-3929
Made in India
Code: TS/DRUGS/16/2014
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 200 mg/38 mg (20 Coated Caplets) Bottle Label
NDC 21130-228-73
Signature
care®
Quality
Guaranteed
IBUPROFEN AND
DIPHENHYDRAMINE CITRATE
TABLETS 200 mg/38 mg
Pain Reliever (NSAID)/ 20 Coated Caplets**
Nighttime Sleep-Aid **Capsule-Shaped Tablets
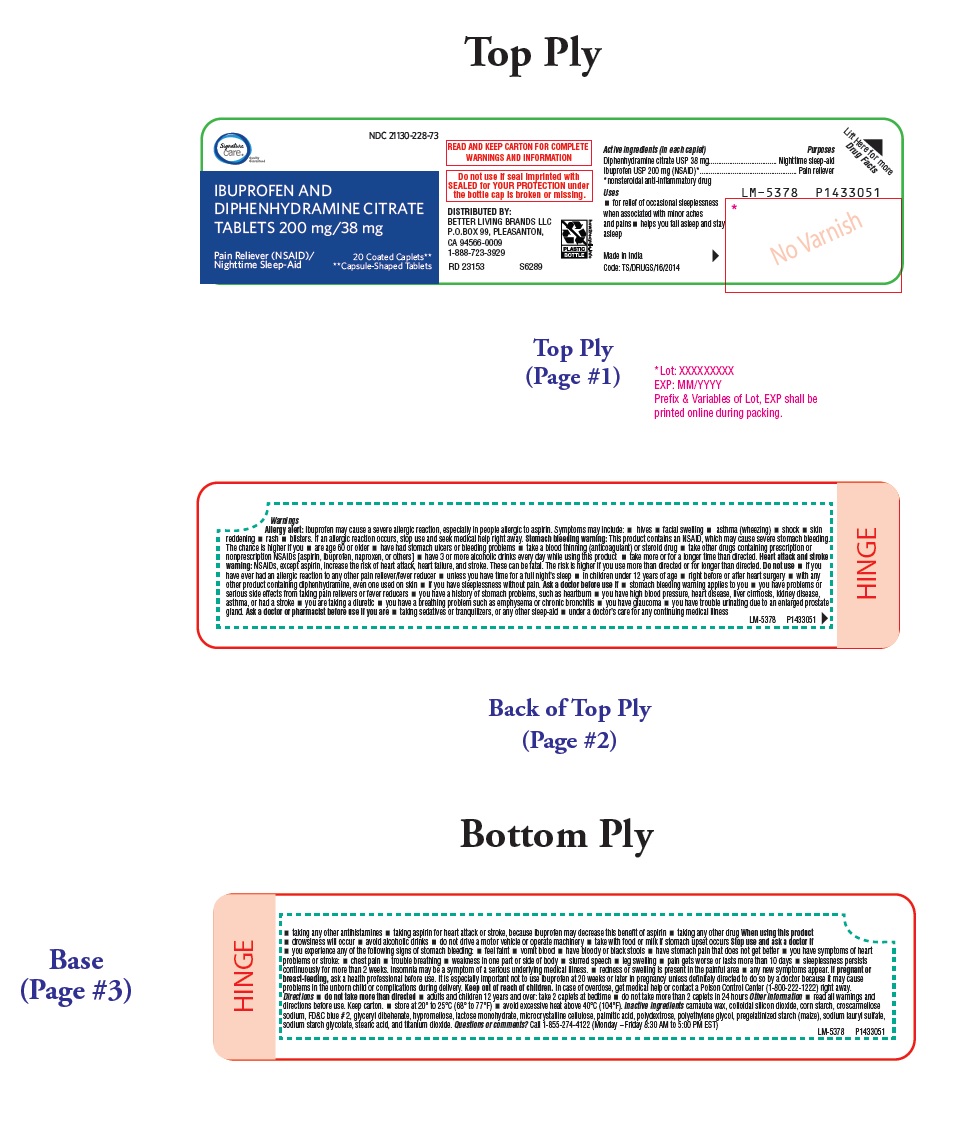
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 200 mg/38 mg (20 Coated Caplets) Bottle Carton Label
Compare to Advil® PM
active ingredients †
Signature
care®
Quality NDC 21130-228-73
Guaranteed
IBUPROFEN AND
DIPHENHYDRAMINE CITRATE
TABLETS 200 mg/38 mg
Actual size
Pain Reliever (NSAID)/ 20 Coated Caplets**
Nighttime Sleep-Aid **Capsule-Shaped Tablets