FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Treatment of Human Smallpox Disease
TPOXX® is indicated for the treatment of human smallpox disease caused by variola virus in adults and pediatric patients weighing at least 3 kg.
1.2 Limitations of Use
The effectiveness of TPOXX for treatment of smallpox disease has not been determined in humans because adequate and well-controlled field trials have not been feasible, and inducing smallpox disease in humans to study the drug’s efficacy is not ethical [see Clinical Studies (14)].
TPOXX efficacy may be reduced in immunocompromised patients based on studies demonstrating reduced efficacy in immunocompromised animal models.
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Instructions
It is recommended that patients 13 kg and above initiate oral treatment with TPOXX capsules if possible. If patients are unable to take oral TPOXX capsules or Drug-Food Preparation, treatment may be initiated with TPOXX injection as a 6 hour intravenous (IV) infusion. If IV treatment is necessary, conversion from IV to oral TPOXX is recommended as soon as oral treatment can be tolerated [see Dosage and Administration (2.3)]. In patients receiving an IV infusion, the first dose of oral treatment should be given at the time of and in place of the next scheduled IV dosing.
In patients receiving an oral treatment who subsequently require IV treatment, the first dose of IV infusion should be given at the time of and in place of the next scheduled oral dosing.
TPOXX capsules
Take TPOXX capsules within 30 minutes after a full meal containing moderate or high fat.
Missed Dose
If a dose of oral TPOXX is missed, the patient should take the dose as soon as possible and anytime up to 8 hours prior to the next scheduled dose. If less than 8 hours remain before the next scheduled dose, do not take the missed dose, and resume dosing at the next scheduled dose.
TPOXX injection
Administer TPOXX injection by IV infusion over 6 hours via an infusion pump. Must dilute TPOXX Injection prior to use [see Dosage and Administration (2.5)].
2.2 Testing Before Initiating and During Treatment with TPOXX Injection
Determine creatinine clearance in all patients before starting TPOXX injection and monitor while receiving TPOXX injection as clinically appropriate [see Dosage and Administration (2.4), Contraindictions (4), Warnings and Precautions (5.2) and Use in Specific Populations (8.4, 8.6)].
2.3 TPOXX Oral Dosage for Pediatric Patients Weighing at Least 13 kg and Adults
The recommended dosage of TPOXX capsules in pediatric patients weighing at least 13 kg and adults is displayed in Table 1 below.
|
aTPOXX capsules should be taken within 30 minutes after a full meal containing moderate or high fat [see Clinical Pharmacology (12.3)] |
||
| Body Weight | Oral Dosage for 14 Daysa | |
| Dosage (Number of Capsules) | Drug Food Preparation for Patients Who Cannot Swallow Capsules | |
| 13 kg to less than 25 kg | 200 mg (1 capsule) every 12 hours | Carefully open the required number of capsules and mix contents of capsule(s) of TPOXX with 30 mL of liquid (e.g., milk, chocolate milk) or soft food (e.g., apple sauce, yogurt). The entire mixture should be administered within 30 minutes of its preparation. |
| 25 kg to less than 40 kg | 400 mg (2 capsules) every 12 hours | |
| 40 kg to less than 120 kg | 600 mg (3 capsules) every 12 hours | |
| 120 kg and above | 600 mg (3 capsules) every 8 hours | |
2.4 Renal Impairment
TPOXX injection is contraindicated in patients with creatinine clearance below 30 mL per minute [see Contraindictions (4)].
2.5 Dosage and Administration of TPOXX Injection for Intravenous Infusion
The recommended dosage of TPOXX injection in pediatric patients weighing at least 3 kg and adults is displayed in Table 2 below.
TPOXX injection is supplied in a single-dose clear glass vial containing 200 mg/20 mL (10 mg/mL). Parenteral drug products must be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. To administer:
- Use aseptic technique when preparing TPOXX injection.
- Withdraw the quantity of TPOXX injection (Table 2), add this volume to the syringe then dilute with two equal parts of either 0.9% (w/v) sodium chloride injection (normal saline) or 5% (w/v) dextrose injection in a syringe of suitable size. Injection with diluents other than 0.9% sodium chloride or 5% dextrose solution has not been studied. NOT FOR IV BOLUS INJECTION. Do not use prefilled infusion bags for product preparation and administration.
- The diluted TPOXX injection may be stored refrigerated (2°C - 8°C) for up to 24 hours or at room temperature (15°C - 25°C) for up to 4 hours.
- Gently swirl the syringe of in-use solution prior to inserting into the syringe pump and infuse over 6 hours every 12 hours for 14 days.
- Do not re-use the single-dose vial once it has been punctured.
|
aPatients weighing at least 13 kg should be switched to TPOXX Capsules to complete the 14 day treatment course as soon as oral therapy can be tolerated. b10 mg/mL TPOXX solution containing 40% hydroxypropyl betadex (8 g per vial) with water for injection [see Dosage Forms and Strengths (3)] cDiluent is either 0.9% (w/v) sodium chloride injection or 5% (w/v) dextrose injection solution. dDepending on size of syringe available with syringe pump system, two separate syringes may be needed for each 6 hour administration. |
|||
| Body Weight | Dosage for up to 14 days | Volume of TPOXX Injectionb | Volume of Diluentc |
| 3 kg to less than 35 kg | 6 mg/kg every 12 hours by intravenous infusion over 6 hoursa | 0.6 mL/kg | 1.2 mL/kg |
| 35 kg to less than 120 kg | 200 mg every 12 hours by intravenous infusion over 6 hours | 20 mL | 40 mL |
| 120 kg and aboved | 300 mg every 12 hours by intravenous infusion over 6 hours | 30 mL | 60 mL |
3 DOSAGE FORMS AND STRENGTHS
TPOXX Capsules
TPOXX capsules are hard gelatin with an opaque orange body imprinted in white ink with “SIGA” followed by the SIGA logo followed by “®”, and an opaque black cap imprinted in white ink with “ST-246®”, containing white to off-white powder. Each capsule contains 200 mg of tecovirimat.
TPOXX Injection
TPOXX injection: 200 mg/20 mL (10 mg/mL) of tecovirimat as a clear, colorless to pale yellow solution in a single-dose vial for further dilution.
4 CONTRAINDICATIONS
TPOXX Capsules:
None.
TPOXX Injection:
The excipient hydroxypropyl-β-cyclodextrin is eliminated through glomerular filtration. Therefore, TPOXX Injection is contraindicated in patients with severe renal impairment (defined as creatinine clearance below 30 mL/min) [see Warnings and Precautions (5.2) and Use in Specific Populations (8.6)].
5 WARNINGS AND PRECAUTIONS
5.1 Hypoglycemia When Co-Administered with Repaglinide
Co-administration of repaglinide and tecovirimat may cause mild to moderate hypoglycemia. Monitor blood glucose and monitor for hypoglycemic symptoms when administering TPOXX with repaglinide [see Drug Interactions (7) and Clinical Pharmacology (12.3)].
In a drug interaction study, 10 of 30 healthy subjects experienced mild (6 subjects) or moderate (4 subjects) hypoglycemia following co-administration of repaglinide (2 mg) and TPOXX. Symptoms resolved in all subjects after intake of food and/or oral glucose.
5.2 Risks of Hydroxypropyl-β-Cyclodextrin Excipient for Patients with Renal Insufficiency and Pediatric Patients < 2 Years of Age
Patients with renal insufficiency
TPOXX Injection: In healthy patients and in patients with mild to severe renal insufficiency, the majority of an 8 g dose of hydroxypropyl-β-cyclodextrin (per 200 mg tecovirimat/20 mL solution) is eliminated in the urine. It is known that clearance of hydroxypropyl-β-cyclodextrin is reduced in patients with mild, moderate, and severe renal impairment, resulting in higher exposure to hydroxypropyl-β-cyclodextrin; in these patients, half-life values are increased over normal values by approximately two-, four-, and six-fold, respectively. In these patients, successive infusions may result in accumulation of hydroxypropyl-β-cyclodextrin until steady state is reached.
In patients with mild (defined as creatinine clearance 60-89 mL/min) and moderate (defined as creatinine clearance 30-59 mL/min) renal impairment, TPOXX Injection should be used with caution. Creatinine clearance should be closely monitored and, if renal toxicity is suspected, consideration should be given to administering TPOXX orally if possible or to using an alternative medication. TPOXX Injection is contraindicated in patients with severe renal impairment (creatinine clearance 30 mL/min) [see Contraindictions (4) and Clinical Pharmacology (12.3)].
Pediatric patients
TPOXX Injection: In pediatric patients less than 2 years of age, there are limited data regarding the use of hydroxypropyl-β-cyclodextrin. Given that renal tubular function rapidly matures over the first few years of life, clearance of hydroxypropyl-β-cyclodextrin may be reduced in young pediatric patients, resulting in higher exposure to hydroxypropyl-β-cyclodextrin. TPOXX Injection should be used with caution in this population given that animal studies have shown potential for nephrotoxicity at very high exposure levels of hydroxypropyl-β-cyclodextrin. Given the potential for drug accumulation due to renal immaturity in pediatric patients less than 2 years, monitoring of renal function after treatment is recommended [see Use in Specific Populations (8.4) and Clinical Pharmacology (12.3)].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of TPOXX has not been studied in patients with smallpox disease.
TPOXX Clinical Trial (Oral Administration)
The safety of TPOXX was evaluated in 359 healthy adult subjects ages 18-79 years in a Phase 3 clinical trial. Of the subjects who received at least one 600 mg dose of TPOXX, 59% were female, 69% were White, 28% were Black/African American, 1% were Asian, and 12% were Hispanic or Latino. Ten percent of the subjects who participated in the study were age 65 or older. Of these 359 subjects, 336 subjects received at least 23 of 28 doses of 600 mg TPOXX in a twice daily (every 12 hours) regimen for 14 days.
Most Frequently Reported Adverse Reactions
The most frequently reported adverse reactions were headache and nausea. Adverse reactions that occurred in at least 2% of subjects in the TPOXX treatment group are shown in Table 3.
|
a Includes abdominal pain, abdominal pain upper, abdominal distension, abdominal discomfort, abdominal pain |
||
| Adverse Reaction | TPOXX 600 mg N = 359 (%) | Placebo N = 90 (%) |
| Headache | 12 | 8 |
| Nausea | 5 | 4 |
| Abdominal paina | 2 | 1 |
| Vomiting | 2 | 0 |
Adverse Reactions Leading to Discontinuation of TPOXX
Six subjects (2%) had their treatment with TPOXX discontinued due to adverse reactions. Each of these subject’s adverse reactions (with severity) is listed below:
- EEG change, abnormal
- Mild upset stomach, dry mouth, decreased concentration and dysphoria
- Mild nausea and fever, moderate diarrhea, severe headache
- Mild palpable purpura
- Mild nausea, fever and chills
- Mild facial redness, facial swelling and pruritus
Less Common Adverse Reactions
Clinically significant adverse reactions that were reported in <2% of subjects exposed to TPOXX and at rates higher than subjects who received placebo are listed below:
- Gastrointestinal: dry mouth, chapped lips, dyspepsia, eructation, oral paresthesia
- General and administration site: pyrexia, pain, chills, malaise, thirst
- Investigations: abnormal electroencephalogram, hematocrit decreased, hemoglobin decreased, heart rate increased
- Musculoskeletal and connective tissue: arthralgia, osteoarthritis
- Nervous system: migraine, disturbance in attention, dysgeusia, paresthesia
- Psychiatric: depression, dysphoria, irritability, panic attack
- Respiratory, Thoracic and Mediastinal Disorders: oropharyngeal pain
- Skin and subcutaneous tissue: palpable purpura, rash, pruritic rash, facial redness, facial swelling, pruritus
TPOXX Clinical Trial (Intravenous Administration)
The safety of multiple doses of 240 mg of TPOXX injection for IV infusion was evaluated in 26 healthy adult subjects ages 23-62 years, inclusive. An additional 6 subjects received placebo. TPOXX injection was administered over a 6 hour period via infusion pump twice daily (every 12 hours) for 7 days. Of the 26 subjects administered TPOXX, 42% were female, 69% were White, 23% were Black/African American, and 42% were Hispanic or Latino.
Most Frequently Reported Adverse Reactions
The most frequently reported adverse reactions included infusion site pain, infusion site swelling, infusion site erythema, infusion site extravasation, and headache. Adverse reactions that occurred in at least 4% of subjects in the TPOXX treatment group are shown in Table 4.
| TPOXX 240 mg N = 26 (%) | Placebo N = 6 (%) |
|
| Infusion Site Pain | 73 | 67 |
| Infusion Site Swelling | 39 | 67 |
| Infusion Site Erythema | 23 | 67 |
| Infusion Site Extravasation | 19 | 50 |
| Headache | 15 | 0 |
Adverse Reactions Leading to Discontinuation of TPOXX Injection
Three subjects (12%) had their treatment with TPOXX injection discontinued due to adverse reactions. One subject had two adverse reactions. Each of these subject’s adverse reactions (with severity) are listed below:
- Moderate Infusion site extravasation
- Mild Infusion site extravasation
- Mild Infusion site swelling and mild infusion site pain
Less Common Adverse Reactions
Clinically significant adverse reactions that were reported in <4% of subjects exposed to TPOXX injection and at rates higher than subjects who received placebo are listed below:
- General and administration site: infusion site discomfort, infusion site edema
- Musculoskeletal and connective tissue: myalgia, arthritis, back pain, muscle tightness
- Gastrointestinal: diarrhea
- Eye: photophobia
- Skin and Subcutaneous Tissue: pruritus generalized
7 DRUG INTERACTIONS
7.1 Effect of TPOXX on Other Drugs
Tecovirimat is a weak inducer of cytochrome P450 (CYP)3A and a weak inhibitor of CYP2C8 and CYP2C19. However, the effects are not expected to be clinically relevant for most substrates of those enzymes based on the magnitude of interactions and the duration of treatment of TPOXX. See Table 5 for clinical recommendations for select sensitive substrates.
7.2 Established Drug Interactions
Table 5 provides a listing of established or significant drug interactions [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
|
a ↓ = decrease, ↑ = increase b These interactions have been studied in healthy adults. |
||
| Concomitant Drug Class: Drug Name | Effect on Concentrationa | Clinical Effect/Recommendation |
| Blood Glucose-Lowering Agent: | ||
| Repaglinideb | ↑ repaglinide | Monitor blood glucose and monitor for hypoglycemic symptoms in patients when TPOXX is co-administered with repaglinide [see Warnings and Precautions (5.1)]. |
| CNS Depressant: | ||
| Midazolamb | ↓ midazolam | Monitor for effectiveness of midazolam. |
7.3 Drugs Without Clinically Significant Interactions With TPOXX
Based on a drug interaction study, no clinically significant drug interactions have been observed when TPOXX is co-administered with bupropion, flurbiprofen, or omeprazole [see Clinical Pharmacology (12.3)].
7.4 Vaccine Interactions
No vaccine-drug interaction studies have been performed in human subjects. Some animal studies have indicated that co-administration of TPOXX at the same time as live smallpox vaccine (vaccinia virus) may reduce the immune response to the vaccine. The clinical impact of this interaction on vaccine efficacy is unknown.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on the use of tecovirimat in pregnant individuals to evaluate for a drug-associated risk of major birth defects, miscarriage, and other adverse maternal and fetal outcomes.
In animal reproduction studies, no embryofetal developmental toxicity was observed in mice during the period of organogenesis at tecovirimat exposures (area under the curve [AUC]) up to 23 times higher than human exposure at the recommended human dose (RHD). In rabbits, no embryofetal developmental toxicity was observed during organogenesis at tecovirimat exposures (AUC) less than human exposures at the RHD. In a mouse pre-/post-natal development study, no toxicities were observed at maternal tecovirimat exposures up to 24 times higher than human exposure at the RHD (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown, and the estimated background risk of miscarriage for the indicated population is higher than the general population. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Tecovirimat was administered orally to pregnant mice at doses up to 1,000 mg/kg/day from gestation Days 6-15. No embryofetal toxicities were observed at doses up to 1,000 mg/kg/day (approximately 23 times higher than human exposure at the RHD).
Tecovirimat was administered orally to pregnant rabbits at doses up to 100 mg/kg/day from gestation Days 6-19. No embryofetal toxicities were observed at doses up to 100 mg/kg/day (0.4 times the human exposure at the RHD).
In the pre-/post-natal development study, tecovirimat was administered orally to pregnant mice at doses up to 1,000 mg/kg/day from gestation Day 6 to post-natal Day 20. No toxicities were observed at doses up to 1,000 mg/kg/day (approximately 24 times higher than human exposure at the RHD).
8.2 Lactation
Risk Summary
Because of the potential for variola virus transmission through direct contact with the breastfed infant, breastfeeding is not recommended in patients with smallpox. There are no data on the presence of tecovirimat in human milk, the effects of the drug on the breastfed infant, or on milk production. Tecovirimat was present in animal milk (see Data). When a drug is present in animal milk, it is likely to be present in human milk.
Data
In a lactation study at doses up to 1,000 mg/kg/day, mean tecovirimat milk to plasma ratios up to approximately 0.8 were observed at 6 and 24 hours post-dose when administered orally to mice on lactation Day 10 or 11.
8.3 Females and Males of Reproductive Potential
Infertility
There are no data on the effect of tecovirimat on human fertility. Decreased fertility due to testicular toxicity was observed in male mice [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
As in adults, the effectiveness of TPOXX in pediatric patients is based solely on efficacy studies in animal models of orthopoxvirus disease. As exposure of healthy pediatric subjects to TPOXX with no potential for direct clinical benefit is not ethical, pharmacokinetic simulation was used to derive dosing regimens that are predicted to provide pediatric patients with exposures comparable to the observed exposure in adults receiving 600 mg orally twice daily (every 12 hours) or 200 mg intravenously twice daily (every 12 hours). The dosage for pediatric patients is based on weight [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
TPOXX Injection:
There are limited data regarding the use of hydroxypropyl-β-cyclodextrin, an ingredient in TPOXX injection, in pediatric patients less than 2 years of age. Given the potential for drug accumulation due to renal immaturity in pediatric patients less than 2 years, monitoring of renal function after treatment is recommended [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)].
8.5 Geriatric Use
Clinical studies of TPOXX did not include sufficient numbers of subjects aged 65 and over to determine whether the safety profile of TPOXX is different in this population compared to younger subjects. Of the 359 subjects in the clinical study of TPOXX, 10% (36/359) were ≥ 65 years of age, and 1% (4/359) were ≥ 75 years of age. No alteration of dosing is needed for patients ≥ 65 years of age [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
TPOXX Capsules:
No dosage adjustment is required for patients with mild, moderate or severe renal impairment or patients with end stage renal disease (ESRD) requiring hemodialysis [see Clinical Pharmacology (12.3)].
TPOXX Injection:
Hydroxypropyl-β-cyclodextrin, an ingredient in TPOXX injection, when administered intravenously, is eliminated through glomerular filtration. No dosage adjustment is required for patients with mild (creatinine clearance 60-89 mL/min) or moderate (creatinine clearance 30-59 mL/min) renal impairment. TPOXX Injection is contraindicated in patients with severe renal impairment (creatinine clearance below 30 mL/min) [see Contraindictions (4)].
8.7 Hepatic Impairment
No dosage adjustment is required for patients with mild, moderate or severe hepatic impairment (Child Pugh Class A, B, or C) [see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
There is no clinical experience with overdosage of TPOXX. In case of overdosage, monitor patients for any signs or symptoms of adverse effects. Hemodialysis will not significantly remove TPOXX in overdosed patients.
11 DESCRIPTION
TPOXX capsules and TPOXX injection contains tecovirimat, an inhibitor of the orthopoxvirus VP37 envelope wrapping protein.
TPOXX (tecovirimat) capsules, for oral use are immediate release capsules containing tecovirimat monohydrate equivalent to 200 mg of tecovirimat for oral administration. The capsules include the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hydroxypropyl methyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. The capsule shell is composed of gelatin, FD&C blue #1, FD&C red #3, FD&C yellow #6, and titanium dioxide.
TPOXX (tecovirimat) injection, for intravenous use is a sterile, colorless to pale yellow solution free of visible particles that is intended for intravenous use following dilution. Tecovirimat injection is available in a single-dose vial containing 200 mg/20 mL (10 mg/mL) of tecovirimat and 8,000 mg (400 mg/mL) of Hydroxypropyl Betadex, NF (hydroxypropyl β-cyclodextrin) and Water for Injection, USP/NF.
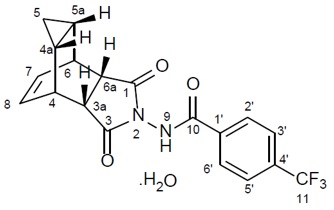
Tecovirimat monohydrate is a white to off-white crystalline solid with the chemical name Benzamide, N-[(3aR,4R,4aR,5aS,6S,6aS)-3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6 ethenocycloprop[f]isoindol-2(1H)-yl]-4-(trifluoromethyl), rel-(monohydrate). The chemical formula is C19H15F3N2O3·H2O representing a molecular weight of 394.35 g/moL. The molecular structure is as follows:
Tecovirimat monohydrate is practically insoluble in water and across the pH range of 2.0-6.5 (< 0.1 mg/mL).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tecovirimat is an antiviral drug against variola (smallpox) virus [see Microbiology (12.4)].
12.2 Pharmacodynamics
Cardiac Electrophysiology
TPOXX does not prolong the QT interval to any clinically relevant extent at the anticipated therapeutic exposure.
12.3 Pharmacokinetics
At the recommended oral dosage of 600 mg every 12 hours administered in healthy adults weighing less than 120 kg, the mean steady-state values of tecovirimat AUC0-24hr, Cmax, and Ctau/trough are 29816 hr•ng/mL (n, CV: 43, 34%), 2159 ng/mL (n, CV: 46, 32%), and 845 ng/mL (n, CV: 45, 47%), respectively. At the recommended intravenous dosage of 200 mg every 12 hours administered by IV infusion over 6 hours in healthy adults, the mean steady-state values of tecovirimat AUC0-24hr, Cmax, and Cmin are 39405 hr•ng/mL (n, CV: 22, 23%), 2630 ng/mL (n, CV: 22, 22%), and 747 ng/mL (n, CV: 22, 29%). Refer to Table 6 for pharmacokinetic parameters of tecovirimat. Tecovirimat steady-state is achieved by Day 4-6.
|
aValue reflects administration of drug with food. bValue refers to mean systemic exposure (AUC24hr). Meal: ~ 600 kcal, ~ 25 g fat.
cTecovirimat is metabolized by hydrolysis of the amide bond and glucuronidation. The following inactive dUridine diphosphate (UDP)-glucuronosyl transferase (UGT) enzymes et1/2 value refers to mean terminal plasma half-life. fSingle dose administration of [14C]-tecovirimat in mass balance study. KEY: NA = Not Applicable or Not Available |
||
| Absorption | 200 mg intravenous | 600 mg oral |
| Median Tmax (h) (Range) | 6 (6-6.5) | 6 (2-24)a |
| Effect of food (relative to fasting) | NA | ↑39%b |
| Distribution | ||
| % Bound to human plasma proteins | 77-82 | |
| Blood-to-plasma ratio (drug or drug-related materials) | 0.62-0.90 | |
| Volume of distribution (Vz or Vz/F, L) (CV%) | 383 (46%) | 1030 |
| Metabolism | ||
| Metabolic pathwaysc | Hydrolysis, UGT1A1d, UGT1A4 | |
| Elimination | ||
| Major route of elimination | Metabolism | |
| Clearance (CL or CL/F, L/hr) (CV%) | 13 (23%) | 31 |
| t1/2 (h)e (CV%) | 21 (45%) | 19 (29%) |
| % of dose excreted in urinef | NA | 73, predominantly as metabolites |
| % of dose excreted in fecesf | NA | 23, predominantly as tecovirimat |
Comparison of Animal and Human PK Data to Support Effective Human Dose Selection
Because the effectiveness of TPOXX cannot be tested in humans, a comparison of tecovirimat exposures achieved in healthy human subjects to those observed in animal models of orthopoxvirus infection (nonhuman primates and rabbits infected with monkeypox virus and rabbitpox virus, respectively) in therapeutic efficacy studies was necessary to support the dosage regimen of 600 mg every 12 hours for treatment of smallpox disease in humans. Humans achieve greater systemic exposure (AUC, Cmax, and Cmin) of tecovirimat following a dose of 600 mg every 12 hours when compared to the therapeutic exposures in these animal models.
Specific Populations
No clinically significant differences in the pharmacokinetics of tecovirimat were observed based on age, sex, ethnicity, renal impairment (based on estimated GFR), or hepatic impairment (Child Pugh Scores A, B or C). At the 600 mg twice-daily dosage, tecovirimat exposure was reduced in adult subjects weighing more than 120 kg compared to the exposures in adult subjects weighing less than 120 kg. Specifically, in 34 adult subjects weighing more than 120 kg who received 600 mg TPOXX orally every 12 hours, the observed mean steady state values of AUC0-24hr, Cmax, and Ctrough were 19500 hr•ng/mL (CV: 23%), 1300 ng/mL (CV: 29%), and 585 ng/mL (CV: 31%), respectively.
Pediatric Patients
TPOXX pharmacokinetics has not been evaluated in pediatric patients. The recommended pediatric dosing regimen is expected to produce tecovirimat exposures that are comparable to those in adult subjects based on a population pharmacokinetic modeling and simulation approach [see Dosage and Administration (2.2) and Use in Specific Populations (8.4)].
Hydroxypropyl-β-cyclodextrin, when administered intravenously, is eliminated through glomerular filtration which may be reduced in pediatric patients with renal immaturity [see Warnings and Precautions (5.2) and Use in Specific Populations (8.4)].
Drug Interaction Studies
The effect of tecovirimat on the exposure of co-administered drugs are shown in Table 7.
|
aAll interaction studies conducted in healthy volunteers with tecovirimat 600 mg twice daily (every 12 hours). bComparison based on exposures when administered as flurbiprofen + omeprazole + midazolam. |
||||
| Co-Administered Drug | Dose of Co-Administered Drug (mg) | N | Mean Ratio (90% CI) of Co-Administered Drug PK With/Without TPOXX No Effect = 1.00 |
|
| Cmax | AUCinf | |||
| Flurbiprofen + omeprazole + midazolamb | omeprazole 20 single dose | 24 | 1.87 (1.51, 2.31) | 1.73 (1.36, 2.19) |
| midazolam 2 single dose | 0.61 (0.54, 0.68) | 0.68 (0.63, 0.73) |
||
| Repaglinide | 2 single dose | 30 | 1.27 (1.12, 1.44) | 1.29 (1.19, 1.40) |
| Bupropion | 150 single dose | 24 | 0.86 (0.79, 0.93) | 0.84 (0.78, 0.89) |
No pharmacokinetic changes were observed for the following drug when co-administered with tecovirimat: flurbiprofen.
Cytochrome P450 (CYP) Enzymes: Tecovirimat is a weak inhibitor of CYP2C8 and CYP2C19, and a weak inducer of CYP3A4. Tecovirimat is not an inhibitor or an inducer of CYP2B6 or CYP2C9.
In Vitro Studies Where Drug Interaction Potential Was Not Further Evaluated Clinically
CYP Enzymes: Tecovirimat is not an inhibitor of CYP1A2, CYP2D6, CYP2E1 or CYP3A4, and is not an inducer of CYP1A2. Tecovirimat is not a substrate for CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 or CYP3A4.
UGT Enzymes: Tecovirimat is a substrate of UGT1A1 and UGT1A4.
Transporter Systems: Tecovirimat inhibited Breast Cancer Resistance Protein (BCRP) in vitro.
Tecovirimat is not an inhibitor of P-glycoprotein (P-gp), organic anion transporting polypeptides 1B1 and 1B3 (OATP1B1 and OATP1B3), organic anion transporter 1 (OAT1), OAT3, and organic cation transporter 2 (OCT2). Tecovirimat is not a substrate for P-gp, BCRP, OATP1B1, and OATP1B3.
12.4 Microbiology
Mechanism of Action
Tecovirimat targets and inhibits the activity of the orthopoxvirus VP37 protein (encoded by and highly conserved in all members of the orthopoxvirus genus) and blocks its interaction with cellular Rab9 GTPase and TIP47, which prevents the formation of egress competent enveloped virions necessary for cell to cell and long range dissemination of virus.
Activity in Cell Culture
In cell culture assays, the effective concentrations of tecovirimat resulting in a 50% reduction in virus-induced cytopathic effect (EC50), were 0.016-0.067 µM, 0.014-0.039 µM, 0.015 µM, and 0.009 µM, for variola, monkeypox, rabbitpox, and vaccinia viruses, respectively. Ranges given for variola and monkeypox viruses are reflective of results from multiple strains assayed.
Non-antagonistic antiviral activity of tecovirimat and brincidofovir against orthopoxviruses has been demonstrated in cell culture and animal models.
Resistance
There are no known instances of naturally occurring tecovirimat resistant orthopoxviruses, although tecovirimat resistance may develop under drug selection. Tecovirimat has a relatively low resistance barrier, and certain amino acid substitutions in the target VP37 protein can confer large reductions in tecovirimat antiviral activity. The possibility of resistance to tecovirimat should be considered in patients who either fail to respond to therapy or who develop recrudescence of disease after an initial period of responsiveness.
Cross Resistance
Cross-resistance between tecovirimat and brincidofovir is not expected based on their distinct mechanisms of action. Where tested, orthopoxvirus isolates resistant to cidofovir (the active metabolite of brincidofovir) have not been resistant to tecovirimat. Likewise, orthopoxvirus isolates resistant to tecovirimat retain their sensitivity to cidofovir.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
Carcinogenicity studies have not been conducted with tecovirimat.
Tecovirimat was not genotoxic in in vitro or in vivo assays, including a bacterial reverse mutation assay, a mammalian mutagenicity assay in mouse lymphoma L5178Y/TK± cells, and in an in vivo mouse micronucleus study.
Impairment of Fertility
In a fertility and early embryonic development study in mice, no effects of tecovirimat on female fertility were observed at tecovirimat exposures (AUC) approximately 24 times higher than human exposure at the RHD. In male mice, decreased male fertility associated with testicular toxicity (increased percent abnormal sperm and decreased sperm motility) was observed at 1,000 mg/kg/day (approximately 24 times the human exposure at the RHD).
13.2 Animal Toxicology and/or Pharmacology
In a repeat-dose toxicology study in dogs, convulsions (tonic and clonic) were observed in one animal within 6 hours of a single dose of 300 mg/kg (approximately 4 times higher than the highest observed human exposure at the RHD based on Cmax). Electroencephalography (EEG) findings in this animal were consistent with seizure activity during the observed convulsions. Tremors, which were considered non-adverse, were observed at 100 mg/kg/dose (similar to the highest observed human exposure at the RHD based on Cmax), although no convulsions or EEG findings were observed at this dose.
14 CLINICAL STUDIES
Overview
The effectiveness of TPOXX for treatment of smallpox disease has not been determined in humans because adequate and well-controlled field trials have not been feasible, and inducing smallpox disease in humans to study the drug’s efficacy is not ethical. Therefore, the effectiveness of TPOXX for treatment of smallpox disease was established based on results of adequate and well-controlled animal efficacy studies of non-human primates and rabbits infected with non-variola orthopoxviruses. Survival rates observed in the animal studies may not be predictive of survival rates in clinical practice.
Study Design
Efficacy studies were conducted in cynomolgus macaques infected with monkeypox virus, and New Zealand white (NZW) rabbits infected with rabbitpox virus. The primary efficacy endpoint for these studies was survival. In non-human primate studies, cynomolgus macaques were lethally challenged intravenously with 5 x 107 plaque-forming units of monkeypox virus; tecovirimat was administered orally once daily at a dose level of 10 mg/kg for 14 days, starting at Day 4, 5 or 6 post-challenge. In rabbit studies, NZW rabbits were lethally challenged intradermally with 1,000 plaque-forming units of rabbitpox virus; tecovirimat was administered orally once daily for 14 days at a dose level of 40 mg/kg, starting at Day 4 post-challenge. The timing of tecovirimat dosing in these studies was intended to assess efficacy when treatment is initiated after animals have developed clinical signs of disease, specifically dermal pox lesions in cynomolgus macaques, and fever in rabbits. Clinical signs of disease were evident in some animals at Day 2-3 post-challenge but were evident in all animals by Day 4 post-challenge. Survival was monitored for 3-6 times the mean time to death for untreated animals in each model.
Study Results
Treatment with tecovirimat for 14 days resulted in statistically significant improvement in survival relative to placebo, except when given to cynomolgus macaques starting at Day 6 post-challenge (Table 8).
|
aDay post-challenge tecovirimat treatment was initiated bp-value is from 1-sided Boschloo Test (with Berger-Boos modification of gamma = 0.000001) compared to placebo cSurvival percentage in tecovirimat treated animals minus survival percentage in placebo treated animals dExact 95% confidence interval based on the score statistic of difference in survival rates eA placebo control group was not included in this study. KEY: NA = Not Applicable |
|||||
| Treatment Initiationa | Survival Percentage (No. survived/n) | p-valueb | Survival Rate Differencec (95% CI)d |
||
| Placebo | Tecovirimat | ||||
| Cynomolgus Macaques | |||||
| Study 1 | Day 4 | 0% (0/7) | 80% (4/5) | 0.0038 | 80% (20.8%, 99.5%) |
| Study 2 | Day 4 | 0% (0/6) | 100% (6/6) | 0.0002 | 100% (47.1%, 100%) |
| Study 3 | Day 4 | 0% (0/3) | 83% (5/6) | 0.0151 | 83% (7.5%, 99.6%) |
| Day 5 | 83% (5/6) | 0.0151 | 83% (7.5%, 99.6%) | ||
| Day 6 | 50% (3/6) | 0.1231 | 50% (-28.3%, 90.2%) | ||
| NZW Rabbits | |||||
| Study 4 | Day 4 | 0% (0/10) | 90% (9/10) | < 0.0001 | 90% (50.3%, 99.8%) |
| Study 5 | Day 4 | NAe | 88% (7/8) | NA | NA |
16 HOW SUPPLIED/STORAGE AND HANDLING
TPOXX Capsule
How Supplied
Each TPOXX capsule contains 200 mg of tecovirimat. TPOXX capsules are hard gelatin with an opaque orange body imprinted in white ink with “SIGA” followed by the SIGA logo followed by “®”, and an opaque black cap imprinted in white ink with "ST-246®", containing white to off-white powder. Each bottle contains 42 capsules (NDC 50072-200-42) with an induction seal and child-resistant cap.
Storage and Handling
Store capsules in the original bottle at 20°C to 25°C (68°F to 77°F); excursions permitted 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature].
TPOXX Injection
How Supplied
TPOXX injection is supplied in a 30 mL single-dose vial as a clear, colorless to pale yellow solution for intravenous administration containing 200 mg/20 mL (10 mg/mL) of tecovirimat (NDC 50072-010-30). This solution is intended for dilution with either 0.9% (w/v) sodium chloride injection or 5% (w/v) dextrose injection solution. The vial stopper is not made with natural rubber latex. The vials are packed in cartons of seven vials. Short-term (up to 24 hours) storage and handling at an ambient temperature is acceptable.
Storage and Handling
Store TPOXX injection in a refrigerator at 2°C to 8°C (36°F to 46°F) Do not freeze.
The diluted solution(s) of TPOXX injection with either 0.9% (w/v) sodium chloride (normal saline) or 5% (w/v) dextrose solution should be used within 4 hours of preparation if stored at room temperature or within 24 hours of preparation if stored at 2°C to 8°C [see Dosage and Administration (2.5)].
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Efficacy Based on Animal Models Alone
Inform patients that the efficacy of TPOXX is based solely on efficacy studies demonstrating a survival benefit in animals and that the effectiveness of TPOXX has not been tested in humans with smallpox disease [see Clinical Studies (14)].
Important Dosage and Administration Information
Advise patients to take TPOXX capsules as directed within 30 minutes of eating a full meal containing moderate or high fat with 6-8 oz. of water [see Clinical Pharmacology (12.3)]. Inform patients to take TPOXX for the entire duration without missing or skipping a dose [see Dosage and Administration (2)].
Inform patients who cannot swallow capsules to refer to the Instructions for Use [see Dosage and Administration (2)].
Drug Interactions
Inform patients that TPOXX may interact with other drugs. Advise patients to report to their healthcare provider the use of other prescription drugs. Co-administration of TPOXX with repaglinide may cause hypoglycemia [see Warnings and Precautions (5.1) and Drug Interactions (7.2)].
TPOXX injection: Hydroxypropyl-β-cyclodextrin, a required component of TPOXX injection, is eliminated through glomerular filtration. Therefore, in patients with severe renal impairment (defined as creatinine clearance below 30 mL/min), the use of TPOXX injection is contraindicated [see Contraindications (4)]. In patients with mild (defined as creatinine clearance 60-89 mL/min) and moderate (defined as creatinine clearance 30-59 mL/min) renal impairment, TPOXX injection should be used with caution [see Warnings and Precautions (5.2) and Use in Specific Populations (8.6)].
Lactation
Instruct individuals with smallpox not to breastfeed their infant because of the risk of passing variola virus to the breastfed infant [see Use in Specific Populations (8.2)].
TPOXX capsules Manufactured by:
Catalent Pharma Solutions, LLC
1100 Enterprise Drive
Winchester, KY 40391
TPOXX injection manufactured by:
Patheon Manufacturing Services LLC
5900 Martin Luther King Jr. Highway
Greenville, NC 27834
Distributed by:
SIGA Technologies, Inc.
4575 SW Research Way, Suite 110
Corvallis, OR 97333
|
This Patient Information has been approved by the U.S. Food and Drug Administration. |
Issued: 05/2022 |
||||
| PATIENT INFORMATION
|
|||||
| TPOXX (Tē-Pox or Tee-pahx)
(tecovirimat) capsules, for oral use | TPOXX (Tē-Pox or Tee-pahx)
(tecovirimat) injection, for intravenous use |
||||
| What is TPOXX?
TPOXX is a prescription medicine used to treat smallpox disease caused by a type of virus called variola virus in adults and children who weigh at least 7 pounds (3 kg).
|
|||||
| Who should not receive TPOXX injection?
Do not receive TPOXX injection if you or your child have severe kidney problems. TPOXX injection contains an ingredient called hydroxypropyl β-cyclodextrin which is cleared from your body through the kidneys. Tell your healthcare provider if you or your child have kidney problems because receiving TPOXX injection may not be right for you or your child. |
|||||
Before taking or receiving TPOXX, tell your healthcare provider about all of your or your child’s medical conditions, including if you or your child:
Using TPOXX with certain other medicines may affect each other causing possible serious side effects. You can ask your healthcare provider or pharmacist for a list of medications that interact with TPOXX. Especially tell your healthcare provider if you take a medicine used to treat type 2 diabetes called repaglinide. Know the medicines you or your child take. Keep a list of your medicines to show your healthcare provider and pharmacist when you get a new medicine. Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take TPOXX with other medicines. |
|||||
How should I take TPOXX?
TPOXX injection is given to you or your child by intravenous (IV) infusion into a vein slowly over 6 hours using an infusion pump by a health care provider. |
|||||
| What are the possible side effects of TPOXX?
TPOXX may cause serious side effects, including:
|
|||||
| • headache
• drowsiness • hunger • feeling jittery or shaky | • dizziness
• confusion • sweating | • weakness
• fast heartbeat • irritability |
|||
|
The most common side effects of TPOXX capsules include: |
|||||
| • headache
• nausea | • stomach pain
• vomiting |
||||
|
The most common side effects of TPOXX injection include: |
|||||
| • reactions at the site of your IV infusion | |||||
|
These are not all the possible side effects of TPOXX. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||||
How should I store TPOXX?
|
|||||
| General information about the safe and effective use of TPOXX.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use TPOXX for a condition for which it was not prescribed. Do not give TPOXX to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about TPOXX that is written for health professionals. |
|||||
| What are the ingredients in TPOXX?
TPOXX capsules: 200 mg Active ingredient: tecovirimat Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hydroxypropyl methyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. The capsule shell is made of gelatin, FD&C blue No. 1, FD&C red No. 3, FD&C yellow No. 6, and titanium dioxide. TPOXX injection: 200 mg in each 20 mL vial Active ingredient: tecovirimat Inactive ingredients: hydroxypropyl β-cyclodextrin and water for injection. TPOXX capsules manufactured by: Catalent Pharma Solutions 1100 Enterprise Drive Winchester, KY 40391 TPOXX injection manufactured by: Patheon Manufacturing Services LLC 5900 Martin Luther King Jr. Highway Greenville, NC 27834 Distributed By: SIGA Technologies, Inc. 4575 SW Research Way, Suite 110 Corvallis, OR 97333 For more information, go to www.SIGA.com or call 1-888-899-3472. |
|||||
| INSTRUCTIONS FOR USE
TPOXX (Tē-Pox or Tee-pahx) (tecovirimat) capsules, for oral use |
| This Instructions for Use contains information on how to prepare and give a dose of TPOXX capsules to children who weigh 28 pounds (13 kg) to less than 88 pounds (40 kg) or adults or children who have trouble swallowing TPOXX capsules whole. |
| Read this Instructions for Use before taking TPOXX capsules. There may be new information. This Instructions for Use does not take the place of talking to your healthcare provider about your medicinal condition or treatment. |
Step 1: Gather the supplies you need to prepare a dose of TPOXX:
|
| Step 2: Find the weight of the person taking the medicine on the TPOXX Dosing Table (see Figure A). |
| Step 3: Find the prescribed dose in the same row as the weight of the person taking the medicine on the TPOXX Dosing Table (see Figure A). |
Step 4:
|
Step 5: Find the number of TPOXX capsules needed in the same row as the weight of the person taking the medicine on the TPOXX Dosing Table (see Figure A).
|
Step 6: Hold the TPOXX capsule in a sideways (horizontal) position directly over the bowl or cup to make sure none of the medicine is lost.
|
Step 7: Use the tablespoon to mix together the capsule contents and the liquid or soft food.
|
Step 8: Swallow the TPOXX medicine mixture.All the TPOXX medicine mixture should be swallowed to make sure the entire dose it taken.
|
|
aGiven twice daily every 12 hours, by mouth, for 14 days. bGiven three times a day every 8 hours, by mouth, for 14 days. |
||||
| TPOXX Dosing Table (Figure A) | ||||
| Body Weight | Prescribed Dose | Amount of Liquid or Soft Food | Number of Capsules | Food and Medicine Mixture Instructions |
| 28 pounds (13 kg) to less than 55 pounds (25 kg)a | 200 mg (1 capsule) Every 12 hours | 2 tablespoons | 1 TPOXX capsule | Mix entire contents of 1 TPOXX capsule with 2 tablespoons of liquid or soft food. |
| 55 pounds (25 kg) to less than 88 pounds (40 kg)a | 400 mg (2 capsules) Every 12 hours | 2 tablespoons | 2 TPOXX capsules | Mix entire contents of 2 TPOXX capsules with 2 tablespoons of liquid or soft food. |
| 88 pounds (40 kg) to less than 265 pounds (120 kg)a | 600 mg (3 capsules) Every 12 hours | 2 tablespoons | 3 TPOXX capsules | Mix entire contents of 3 TPOXX capsules with 2 tablespoons of liquid or soft food. |
| 265 pounds (120 kg) and aboveb | 600 mg (3 capsules) Every 8 hours | 2 tablespoons | 3 TPOXX capsules | Mix entire contents of 3 TPOXX capsules with 2 tablespoons of liquid or soft food. |
How should I store TPOXX Capsules?
- Store TPOXX at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep TPOXX in its original container.
Keep TPOXX and all medicines out of the reach of children.
TPOXX capsules Manufactured by:
Catalent Pharma Solutions
1100 Enterprise Drive
Winchester, KY 40391
TPOXX injection manufactured by:
Patheon Manufacturing Services LLC
5900 Martin Luther King Jr. Highway
Greenville, NC 27834
Distributed by:
SIGA Technologies, Inc.
4575 SW Research Way, Suite 110
Corvallis, OR 97333
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 05/2022