WARNINGS
Personnel and Equipment for Monitoring and Depression
Midazolam HCl syrup has been associated with respiratory depression and respiratory arrest, especially when used for sedation in noncritical care settings. Midazolam HCl syrup has been associated with reports of respiratory depression, airway obstruction, desaturation, hypoxia, and apnea, most often when used concomitantly with other central nervous system depressants. Midazolam HCl Syrup should be used only in hospital or ambulatory care settings, including physicians’ and dentists’ offices, that can provide for continuous monitoring of respiratory and cardiac function. Immediate availability of resuscitative drugs and age- and size-appropriate equipment for ventilation and intubation, and personnel trained in their use and skilled in airway management should be assured
[See
WARNINGS]
. For deeply sedated patients, a dedicated individual, other than the practitioner performing the procedure, should monitor the patient throughout the procedure.
Risks From Concomitant Use With Opioids
Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Monitor patients for respiratory depression and sedation
[see
WARNINGS,
PRECAUTIONS/Drug Interactions]
.
DESCRIPTION
Midazolam is a benzodiazepine available as midazolam HCl syrup for oral administration. Midazolam, a white to light yellow crystalline compound, is insoluble in water, but can be solubilized in aqueous solutions by formation of the hydrochloride salt in situ under acidic conditions. Chemically, midazolam HCl is 8-chloro-6-(2-fluorophenyl)-1-methyl-4 H-imidazo[1,5-a][1,4]benzodiazepine hydrochloride.
Midazolam hydrochloride has the molecular formula C 18H 13ClFN 3·HCl, a calculated molecular weight of 362.25 and the following structural formula:
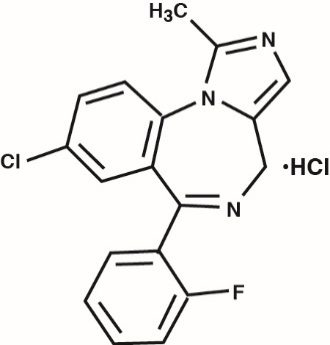
Each mL of the syrup contains midazolam hydrochloride equivalent to 2 mg midazolam compounded with bitterness modifier, artificial cherry-brandy flavor, citric acid anhydrous, D&C Red #33, edetate disodium, glycerin, sodium benzoate, sodium citrate, sodium saccharin, sorbitol solution, and water; the pH is adjusted to 2.8 to 3.6 with hydrochloric acid.
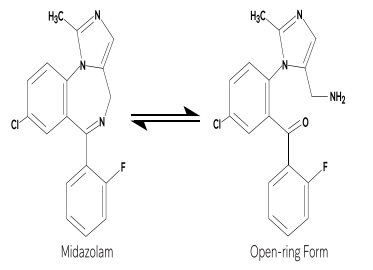
Under the acidic conditions required to solubilize midazolam in the syrup, midazolam is present as an equilibrium mixture (shown below) of the closed ring form shown above and an open-ring structure formed by the acid-catalyzed ring opening of the 4,5-double bond of the diazepine ring. The amount of open-ring form is dependent upon the pH of the solution. At the specified pH of the syrup, the solution may contain up to about 40% of the open-ring compound. At the physiologic conditions under which the product is absorbed (pH of 5 to 8) into the systemic circulation, any open-ring form present reverts to the physiologically active, lipophilic, closed-ring form (midazolam) and is absorbed as such.
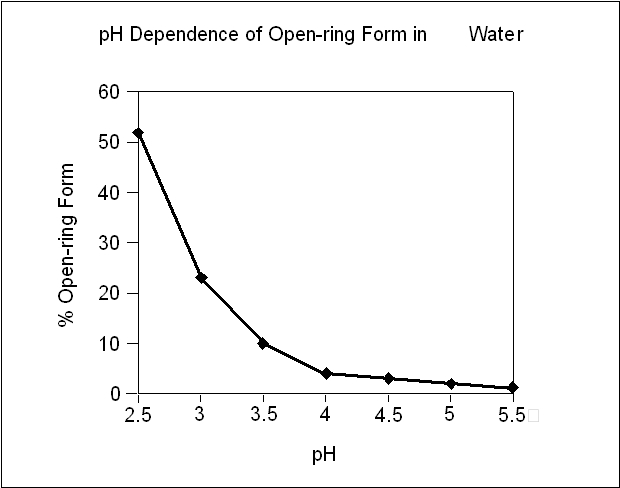
The following chart below plots the percentage of midazolam present as the open-ring form as a function of pH in aqueous solutions. As indicated in the graph, the amount of open-ring compound present in solution is sensitive to changes in pH over the pH range specified for the product: 2.8 to 3.6. Above pH 5, at least 99% of the mixture is present in the closed-ring form.
CLINICAL PHARMACOLOGY
Midazolam is a short-acting benzodiazepine central nervous system (CNS) depressant.
Pharmacodynamics
Pharmacodynamic properties of midazolam and its metabolites, which are similar to those of other benzodiazepines, include sedative, anxiolytic, amnesic and hypnotic activities. Benzodiazepine pharmacologic effects appear to result from reversible interactions with the γ-amino butyric acid (GABA) benzodiazepine receptor in the CNS, the major inhibitory neurotransmitter in the central nervous system. The action of midazolam is readily reversed by the benzodiazepine receptor antagonist, flumazenil.
Data from published reports of studies in pediatric patients clearly demonstrate that oral midazolam provides safe and effective sedation and anxiolysis prior to surgical procedures that require anesthesia as well as before other procedures that require sedation but may not require anesthesia. The most commonly reported effective doses range from 0.25 to 1.0 mg/kg in children (6 months to <16 years). The single most commonly reported effective dose is 0.5 mg/kg. Time to onset of effect is most frequently reported as 10 to 20 minutes.
The effects of midazolam on the CNS are dependent on the dose administered, the route of administration, and the presence or absence of other medications.
Following premedication with oral midazolam, time to recovery has been assessed in pediatric patients using various measures, such as time to eye opening, time to extubation, time in the recovery room, and time to discharge from the hospital. Most placebo-controlled trials (8 total) have shown little effect of oral midazolam on recovery time from general anesthesia; however, a number of other placebo-controlled studies (5 total) have demonstrated some prolongation in recovery time following premedication with oral midazolam. Prolonged recovery may be related to duration of the surgical procedure and/or use of other medications with central nervous system depressant properties.
Partial or complete impairment of recall following oral midazolam has been demonstrated in several studies. Amnesia for the surgical experience was greater after oral midazolam when used as a premedicant than after placebo and was generally considered a benefit. In one study, 69% of midazolam patients did not remember mask application versus 6% of placebo patients.
Episodes of oxygen desaturation, respiratory depression, apnea, and airway obstruction have been reported in <1% of pediatric patients following premedication (eg, sedation prior to induction of anesthesia) with midazolam HCl syrup; the potential for such adverse events are markedly increased when oral midazolam is combined with other central nervous system depressing agents and in patients with abnormal airway anatomy, patients with cyanotic congenital heart disease, or patients with sepsis or severe pulmonary disease [see WARNINGS] .
Concomitant use of barbiturates or other central nervous system depressants may increase the risk of hypoventilation, airway obstruction, desaturation or apnea, and may contribute to profound and/or prolonged drug effect. In one study of pediatric patients undergoing elective repair of congenital cardiac defects, premedication regimens (oral dose of 0.75 mg/kg midazolam or IM morphine plus scopolamine) increased transcutaneous carbon dioxide (PtcCO 2), decreased SpO 2 (as measured by pulse oximetry), and decreased respiratory rates preferentially in patients with pulmonary hypertension. This suggests that hypercarbia or hypoxia following premedication might pose a risk to children with congenital heart disease and pulmonary hypertension. In a study of an adult population 65 years and older, the preinduction administration of oral midazolam 7.5 mg resulted in a 60% incidence of hypoxemia (paO 2 <90% for over 30 seconds) at some time during the operative procedure versus 15% for the nonpremedicated group.
Pharmacokinetics
Absorption: Midazolam is rapidly absorbed after oral administration and is subject to substantial intestinal and hepatic first-pass metabolism. The pharmacokinetics of midazolam and its major metabolite, α-hydroxymidazolam, and the absolute bioavailability of midazolam HCl syrup were studied in pediatric patients of different ages (6 months to <16 years old) over a 0.25 to 1.0 mg/kg dose range. Pharmacokinetic parameters from this study are presented in Table 1. The mean T max values across dose groups (0.25, 0.5, and 1.0 mg/kg) range from 0.17 to 2.65 hours. Midazolam exhibits linear pharmacokinetics between oral doses of 0.25 to 1.0 mg/kg (up to a maximum dose of 40 mg) across the age groups ranging from 6 months to <16 years. Linearity was also demonstrated across the doses within the age group of 2 years to <12 years having 18 patients at each of the three doses. The absolute bioavailability of the midazolam HCl syrup in pediatric patients is about 36%, which is not affected by pediatric age or weight. The AUC 0-∞ ratio of α-hydroxymidazolam to midazolam for the oral dose in pediatric patients is higher than for an IV dose (0.38 to 0.75 versus 0.21 to 0.39 across the age group of 6 months to <16 years), and the AUC 0-∞ ratio of α-hydroxymidazolam to midazolam for the oral dose is higher in pediatric patients than in adults (0.38 to 0.75 versus 0.40 to 0.56).
Food effect has not been tested using midazolam HCl syrup. When a 15 mg oral tablet of midazolam was administered with food to adults, the absorption and disposition of midazolam was not affected. Feeding is generally contraindicated prior to sedation of pediatric patients for procedures.
|
Number of subjects/age group |
Dose (mg/kg) |
T max (h) |
C max (ng/mL) |
t 1/2 (h) |
AUC 0-∞ (ng•h/mL) |
|
6 months to <2 years old |
|||||
|
1 |
0.25 |
0.17 |
28.0 |
5.82 |
67.6 |
|
1 |
0.50 |
0.35 |
66.0 |
2.22 |
152 |
|
1 |
1.00 |
0.17 |
61.2 |
2.97 |
224 |
|
2 to <12 years old |
|||||
|
18 |
0.25 |
0.72 ± 0.44 |
63.0 ± 30.0 |
3.16 ± 1.50 |
138 ± 89.5 |
|
18 |
0.50 |
0.95 ± 0.53 |
126 ± 75.8 |
2.71 ± 1.09 |
306 ± 196 |
|
18 |
1.00 |
0.88 ± 0.99 |
201 ± 101 |
2.37 ± 0.96 |
743 ± 642 |
|
12 to <16 years old |
|||||
|
4 |
0.25 |
2.09 ± 1.35 |
29.1 ± 8.2 |
6.83 ± 3.84 |
155 ± 84.6 |
|
4 |
0.50 |
2.65 ± 1.58 |
118 ± 81.2 |
4.35 ± 3.31 |
821 ± 568 |
|
2 |
1.00 |
0.55 ± 0.28 |
191 ± 47.4 |
2.51 ± 0.18 |
566 ± 15.7 |
Distribution: The extent of plasma protein binding of midazolam is moderately high and concentration independent. In adults and pediatric patients older than 1 year, midazolam is approximately 97% bound to plasma protein, principally albumin. In healthy volunteers, α-hydroxymidazolam is bound to the extent of 89%. In pediatric patients (6 months to <16 years) receiving 0.15 mg/kg IV midazolam, the mean steady-state volume of distribution ranged from 1.24 to 2.02 L/kg.
Metabolism: Midazolam is primarily metabolized in the liver and gut by human cytochrome P450 IIIA4 (CYP3A4) to its pharmacologic active metabolite, α-hydroxymidazolam, followed by glucuronidation of the α-hydroxyl metabolite which is present in unconjugated and conjugated forms in human plasma. The α-hydroxymidazolam glucuronide is then excreted in urine. In a study in which adult volunteers were administered intravenous midazolam (0.1 mg/kg) and α-hydroxymidazolam (0.15 mg/kg), the pharmacodynamic parameter values of the maximum effect (E max) and concentration eliciting half-maximal effect (EC 50) were similar for both compounds. The effects studied were reaction time and errors in tracing tests. The results indicate that α-hydroxymidazolam is equipotent and equally effective as unchanged midazolam on a total plasma concentration basis. After oral or intravenous administration, 63% to 80% of midazolam is recovered in urine as α-hydroxymidazolam glucuronide. No significant amount of parent drug or metabolites is extractable from urine before beta-glucuronidase and sulfatase deconjugation, indicating that the urinary metabolites are excreted mainly as conjugates.
Midazolam is also metabolized to two other minor metabolites: 4-hydroxy metabolite (about 3% of the dose) and 1,4-dihydroxy metabolite (about 1% of the dose) are excreted in small amounts in the urine as conjugates.
Elimination: The mean elimination half-life of midazolam ranged from 2.2 to 6.8 hours following single oral doses of 0.25, 0.5, and 1.0 mg/kg of midazolam HCl syrup. Similar results (ranged from 2.9 to 4.5 hours) for the mean elimination half-life were observed following IV administration of 0.15 mg/kg of midazolam to pediatric patients (6 months to <16 years old). In the same group of patients receiving the 0.15 mg/kg IV dose, the mean total clearance ranged from 9.3 to 11.0 mL/min/kg.
Pharmacokinetic-Pharmacodynamic Relationships: The relationship between plasma concentration and sedation and anxiolysis scores of midazolam HCl syrup (single oral doses of 0.25, 0.5, or 1.0 mg/kg) was investigated in three age groups of pediatric patients (6 months to <2 years, 2 to <12 years, and 12 to <16 years old). In this study, the patient’s sedation scores were recorded at baseline and at 10-minute intervals up to 30 minutes after oral dosing until satisfactory sedation (“drowsy” or “asleep but responsive to mild shaking” or “asleep and not responsive to mild shaking”) was achieved. Anxiolysis scores were measured at the time when the patient was separated from his/her parents and at mask induction. The results of the analyses showed that the mean midazolam plasma concentration as well as the mean of midazolam plus α-hydroxymidazolam for those patients with a sedation score of 4 (asleep but responsive to mild shaking) is significantly different than the mean concentrations for those patients with a sedation score of 3 (drowsy), which is significantly different than the mean concentrations for patients with a sedation score of 2 (awake/calm). The statistical analysis indicates that the greater the midazolam, or midazolam plus α-hydroxymidazolam concentration, the greater the maximum sedation score for pediatric patients. No such trend was observed between anxiolysis scores and the mean midazolam concentration or mean of midazolam plus α-hydroxymidazolam concentration, however, anxiolysis is a more variable surrogate measurement of clinical response.
Special Populations:Renal Impairment: Although the pharmacokinetics of intravenous midazolam in adult patients with chronic renal failure differed from those of subjects with normal renal function, there were no alterations in the distribution, elimination, or clearance of unbound drug in the renal failure patients. However, the effects of renal impairment on the active metabolite α-hydroxymidazolam are unknown.
Hepatic Dysfunction: Chronic hepatic disease alters the pharmacokinetics of midazolam. Following oral administration of 15 mg of midazolam HCl syrup, C max and bioavailability values were 43% and 100% higher, respectively, in adult patients with hepatic cirrhosis than adult subjects with normal liver function. In the same patients with hepatic cirrhosis, following IV administration of 7.5 mg of midazolam, the clearance of midazolam was reduced by about 40% and the elimination half-life was increased by about 90% compared with subjects with normal liver function. Midazolam should be titrated for the desired effect in patients with chronic hepatic disease.
Congestive Heart Failure: Following oral administration of 7.5 mg of midazolam HCl syrup, elimination half-life values were 43% higher in adult patients with congestive heart failure than in control subjects.
Neonates: Midazolam HCl syrup has not been studied in pediatric patients less than 6 months of age.
Drug-Drug Interactions: See PRECAUTIONS: Drug Interactions.
Inhibitors of CYP3A4 Isozymes: Table 2 summarizes the changes in the C max and AUC of midazolam when drugs known to inhibit CYP3A4 were concurrently administered with oral midazolam HCl syrup in adult subjects.
|
Interacting Drug |
Adult Doses Studied |
% Increase in C max of Oral Midazolam |
% Increase in AUC of Oral Midazolam |
|
Cimetidine |
800 to 1,200 mg up to 4 times a day in divided doses |
6 to 138 |
10 to 102 |
|
Diltiazem |
60 mg three times a day |
105 |
275 |
|
Erythromycin |
500 mg three times a day |
170 to 171 |
281 to 341 |
|
Fluconazole |
200 mg daily |
150 |
250 |
|
Grapefruit Juice |
200 mL |
56 |
52 |
|
Itraconazole |
100 to 200 mg daily |
80 to 240 |
240 to 980 |
|
Ketoconazole |
400 mg daily |
309 |
1,490 |
|
Ranitidine |
150 mg two or three times a day; 300 mg daily |
15 to 67 |
9 to 66 |
|
Roxithromycin |
300 mg daily |
37 |
47 |
|
Saquinavir |
1,200 mg three times a day |
235 |
514 |
|
Verapamil |
80 mg three times a day |
97 |
192 |
Other drugs known to inhibit the effects of CYP3A4, such as protease inhibitors, would be expected to have similar effects on these midazolam pharmacokinetic parameters.
Inducers of CYP3A4 Isozymes: Table 3 summarizes the changes in the C max and AUC of midazolam when drugs known to induce CYP3A4 were concurrently administered with midazolam HCl syrup in adult subjects. The clinical significance of these changes is unclear.
|
Interacting Drug |
Adult Doses Studied |
% Decrease in C max of Oral Midazolam |
% Decrease in AUC of Oral Midazolam |
|
Carbamazepine |
Therapeutic Doses |
93 |
94 |
|
Phenytoin |
Therapeutic Doses |
93 |
94 |
|
Rifampin |
600 mg/day |
94 |
96 |
Although not tested, phenobarbital, rifabutin and other drugs known to induce the effects of CYP3A4 would be expected to have similar effects on these midazolam pharmacokinetic parameters.
Drugs that did not affect midazolam pharmacokinetics are presented in Table 4.
|
Interacting Drug |
Adult Doses Studied |
|
Azithromycin |
500 mg/day |
|
Nitrendipine |
20 mg |
|
Terbinafine |
200 mg/day |
Clinical Trials:Dose Ranging, Safety and Efficacy Study with Midazolam Hydrochloride Syrup in Pediatric Patients: The effectiveness of midazolam HCl syrup as a premedicant to sedate and calm pediatric patients prior to induction of general anesthesia was compared among three different doses in a randomized, double-blind, parallel-group study. Patients of ASA physical status I, II or III were stratified to 1 of 3 age groups (6 months to <2 years, 2 to <6 years, and 6 to <16 years), and within each age group randomized to 1 of 3 dosing groups (0.25, 0.5, and 1.0 mg/kg up to a maximum dose of 20 mg). Greater than 90% of treated patients achieved satisfactory sedation and anxiolysis at least one timepoint within 30 minutes posttreatment. Similarly high proportions of patients exhibited satisfactory ease of separation from parent or guardian and were cooperative at the time of mask induction with nitrous oxide and halothane administration. Onset time of satisfactory sedation or anxiolysis occurred within 10 minutes after treatment for >70% of patients who started with an unsatisfactory baseline rating. Whereas pairwise comparisons (0.25 mg/kg versus 0.5 mg/kg groups, and 0.5 mg/kg versus 1.0 mg/kg groups) on satisfactory sedation did not yield significant p-values (p=0.08 in both cases), comparative analysis of the clinical response between the high and low doses demonstrated that a higher proportion of patients in the 1.0 mg/kg dose group exhibited satisfactory sedation and anxiolysis as compared to the 0.25 mg/kg group (p<0.05).
INDICATIONS AND USAGE
Midazolam HCl syrup is indicated for use in pediatric patients for sedation, anxiolysis and amnesia prior to diagnostic, therapeutic or endoscopic procedures or before induction of anesthesia.
Midazolam HCl syrup is intended for use in monitored settings only and not for chronic or home use [see Warnings].
CONTRAINDICATIONS
Midazolam HCl syrup is contraindicated in patients with a known hypersensitivity to the drug or allergies to cherries or formulation excipients. Benzodiazepines are contraindicated in patients with acute narrow-angle glaucoma. Benzodiazepines may be used in patients with open-angle glaucoma only if they are receiving appropriate therapy. Measurements of intraocular pressure in patients without eye disease show a moderate lowering following induction of general anesthesia with injectable midazolam; patients with glaucoma have not been studied.
WARNINGS
Personnel and Equipment for Monitoring and Resuscitation
Midazolam HCl syrup should be used only in hospital or ambulatory care settings, including physicians’ and dentists’ offices, that are equipped to provide continuous monitoring of respiratory and cardiac function. Midazolam HCl syrup must only be administered to patients if they will be monitored by direct visual observation by a health care professional. If midazolam HCl syrup will be administered in combination with other anesthetic drugs or drugs which depress the central nervous system, patients must be monitored by persons specifically trained in the use of these drugs and, in particular, in the management of respiratory effects of these drugs, including respiratory and cardiac resuscitation of patients in the age group being treated.
For deeply sedated patients, a dedicated individual whose sole responsibility is to observe the patient, other than the practitioner performing the procedure, should monitor the patient throughout the procedure.
Patients should be continuously monitored for early signs of hypoventilation, airway obstruction, or apnea with means for detection readily available (eg, pulse oximetry). Hypoventilation, airway obstruction, and apnea can lead to hypoxia and/or cardiac arrest unless effective countermeasures are taken immediately. The immediate availability of specific reversal agents (flumazenil) is highly recommended. Vital signs should continue to be monitored during the recovery period. Because midazolam can depress respiration [see CLINICAL PHARMACOLOGY] , especially when used concomitantly with opioid agonists and other sedatives [see DOSAGE AND ADMINISTRATION] , it should be used for sedation/anxiolysis/amnesia only in the presence of personnel skilled in early detection of hypoventilation, maintaining a patent airway, and supporting ventilation.
Episodes of oxygen desaturation, respiratory depression, apnea, and airway obstruction have been occasionally reported following premedication (sedation prior to induction of anesthesia) with oral midazolam; such events are markedly increased when oral midazolam is combined with other central nervous system depressing agents and in patients with abnormal airway anatomy, patients with cyanotic congenital heart disease, or patients with sepsis or severe pulmonary disease.
Risks from Concomitant Use with Opioids
Concomitant use of benzodiazepines, including midazolam, and opioids may result in profound sedation, respiratory depression, coma and death. If a decision is made to use midazolam concomitantly with opioids, monitor patients for respiratory depression and sedation [see PRECAUTIONS/Drug Interactions] .
Risk of Respiratory Adverse Events
Serious respiratory adverse events have occurred after administration of oral midazolam, most often when midazolam was used in combination with other central nervous system depressants. These adverse events have included respiratory depression, airway obstruction, oxygen desaturation, apnea, and rarely, respiratory and/or cardiac arrest [see BOX WARNING] . When oral midazolam is administered as the sole agent at recommended doses respiratory depression, airway obstruction, oxygen desaturation, and apnea occur infrequently [see DOSAGE AND ADMINISTRATION] .
Prior to the administration of midazolam in any dose, the immediate availability of oxygen, resuscitative drugs, age- and size-appropriate equipment for bag/valve/mask ventilation and intubation, and skilled personnel for the maintenance of a patent airway and support of ventilation should be ensured.
Individualization of Dosage
Midazolam HCl syrup must never be used without individualization of dosage, particularly when used with other medications capable of producing central nervous system depression. See DOSAGE AND ADMINISTRATIONfor complete information.
Other Adverse Events
Reactions such as agitation, involuntary movements (including tonic/clonic movements and muscle tremor), hyperactivity and combativeness have been reported in both adult and pediatric patients. Consideration should be given to the possibility of paradoxical reaction. Should such reactions occur, the response to each dose of midazolam and all other drugs, including local anesthetics, should be evaluated before proceeding. Reversal of such responses with flumazenil has been reported in pediatric and adult patients.
Concomitant Use of Central Nervous System Depressants
Concomitant use of barbiturates, alcohol or other central nervous system depressants may increase the risk of hypoventilation, airway obstruction, desaturation, or apnea and may contribute to profound and/or prolonged drug effect. Narcotic premedication also depresses the ventilatory response to carbon dioxide stimulation.
Drug-Drug Interactions
Coadministration of oral midazolam in patients who are taking ketoconazole and intraconazole, and saquinavir has been shown to result in large increases in C max and AUC of midazolam due to a decrease in plasma clearance of midazolam [see CLINICAL PHARMACOLOGY: Pharmacokinetics: Special Populations: Drug-Drug Interactions and PRECAUTIONS]. Due to the potential for intense and prolonged sedation and respiratory depression, midazolam HCl syrup should only be coadministered with these medications if absolutely necessary and with appropriate equipment and personnel available to respond to respiratory insufficiency.
Debilitation and Comorbidity Considerations
Higher risk pediatric surgical patients may require lower doses, whether or not concomitant sedating medications have been administered. Pediatric patients with cardiac or respiratory compromise may be unusually sensitive to the respiratory depressant effect of midazolam. Pediatric patients undergoing procedures involving the upper airway such as upper endoscopy or dental care, are particularly vulnerable to episodes of desaturation and hypoventilation due to partial airway obstruction. Patients with chronic renal failure and patients with congestive heart failure eliminate midazolam more slowly [see CLINICAL PHARMACOLOGY] .
Return to Cognitive Function
Midazolam is associated with a high incidence of partial or complete impairment of recall for the next several hours. The decision as to when patients who have received midazolam HCl syrup, particularly on an outpatient basis, may again engage in activities requiring complete mental alertness, operate hazardous machinery or drive a motor vehicle must be individualized. Gross tests of recovery from the effects of midazolam HCl syrup [see CLINICAL PHARMACOLOGY] cannot be relied upon to predict reaction time under stress. It is recommended that no patient operate hazardous machinery or a motor vehicle until the effects of the drug, such as drowsiness, have subsided or until one full day after anesthesia and surgery, whichever is longer. Particular care should be taken to assure safe ambulation.
Neonatal Sedation and Withdrawal Syndrome
Use of midazolam HCl syrup late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate [see PRECAUTIONS: Pregnancy] . Monitor neonates exposed to midazolam HCl syrup during pregnancy or labor for signs of sedation and monitor neonates exposed to midazolam HCl syrup during pregnancy for signs of withdrawal; manage these neonates accordingly.
Usage in Preterm Infants and Neonates
Midazolam HCl syrup has not been studied in patients less than 6 months of age.
Pediatric Neurotoxicity
Published animal studies demonstrate that the administration of anesthetic and sedation drugs that block NMDA receptors and/or potentiate GABA activity increase neuronal apoptosis in the developing brain and result in long-term cognitive deficits when used for longer than 3 hours. The clinical significance of these findings in not clear. However, based on the available date, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester of gestation through the first several months of life, but may extend out to approximately three years of age in humans [seePRECAUTIONS; Pregnancy, Pediatric Use and ANIMAL PHARMACOLOGY AND/OR TOXICOLOGY] .
Some published studies in children suggest that similar deficits may occur after repeated or prolonged exposures to anesthetic agents early in life and may result in adverse cognitive or behavioral effects. These studies have substantial limitations, and it is not clear if the observed effects are due to the anesthetic/sedation drug administration or other factors such as the surgery or underlying illness.
Anesthetic and sedation drugs are a necessary part of the care of children and pregnant women needing surgery, other procedures, or tests that cannot be delayed, and no specific medications have been shown to be safer than any other. Decisions regarding the timing of any elective procedures requiring anesthesia should take into consideration the benefits of the procedure weighed against the potential risks.
PRECAUTIONS
General
The efficacy and safety of midazolam in clinical use are functions of the dose administered, the clinical status of the individual patient, and the use of concomitant medications capable of depressing the CNS. Anticipated effects may range from mild sedation to deep levels of sedation with a potential loss of protective reflexes, particularly when coadministered with anesthetic agents or other CNS depressants. Care must be taken to individualize the dose of midazolam HCl syrup based on the patient’s age, underlying medical/surgical conditions, concomitant medications, and to have the personnel, age- and size-appropriate equipment and facilities available for monitoring and intervention. Practitioners administering midazolam must have the skills necessary to manage reasonably foreseeable adverse effects, particularly skills in airway management.
Information for Patients
To assure safe and effective use of midazolam HCl syrup, the following information and instructions should be communicated to the patient when appropriate:
- Inform your physician about any alcohol consumption and medicine you are now taking, especially blood pressure medication, antibiotics, and protease inhibitors, including drugs you buy without a prescription. Alcohol has an increased effect when consumed with benzodiazepines; therefore, caution should be exercised regarding simultaneous ingestion of alcohol during benzodiazepine treatment.
- Inform your physician if you are pregnant or are planning to become pregnant.
- Inform your physician if you are nursing.
- Patients should be informed of the pharmacological effects of midazolam HCl syrup, such as sedation and amnesia, which in some patients may be profound. The decision as to when patients who have received midazolam HCl syrup, particularly on an outpatient basis, may again engage in activities requiring complete mental alertness, operate hazardous machinery or drive a motor vehicle must be individualized.
- Midazolam should not be taken in conjunction with grapefruit juice.
- For pediatric patients, particular care should be taken to assure safe ambulation.
- Effect of Anesthetic and Sedation Drugs on Early Brain Development: Studies conducted in young animals and children suggest repeated or prolonged use of general anesthetic or sedation drugs in children younger than 3 years may have negative effects on their developing brains. Discuss with parents and caregivers the benefits, risks, and timing and duration of surgery or procedures requiring anesthetic and sedation drugs.
Pregnancy: Advise pregnant females that use of midazolam HCl syrup late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in newborns [see WARNINGS: Neonatal Sedation and Withdrawal Syndrome and PRECAUTIONS: Pregnancy] .
Instruct patients to inform their healthcare provider if they are pregnant.
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to midazolam HCl syrup during pregnancy [see PRECAUTIONS, Pregnancy] .
Nursing: Instruct patients to notify their healthcare provider if they are breastfeeding or intend to breastfeed. Instruct breastfeeding patients receiving midazolam to monitor infants for excessive sedation, poor feeding, and poor weight gain, and to seek medical attention if they notice these signs. A lactating woman may consider pumping and discarding breastmilk for at least 4 to 8 hours after receiving midazolam for sedation or anesthesia to minimize drug exposure to a breastfed infant [see PRECAUTIONS, Nursing Mothers] .
Drug Interactions
Effect of Concomitant Use of Benzodiazepines and Opioids: The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABA A sites, and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Monitor patients closely for respiratory depression and sedation.
Other CNS Depressants: One case was reported of inadequate sedation with chloral hydrate and later with oral midazolam due to a possible interaction with methylphenidate administered chronically in a 2-year-old boy with a history of Williams syndrome. The difficulty in achieving adequate sedation may have been the result of decreased absorption of the sedatives due to both the gastrointestinal effects and stimulant effects of methylphenidate.
The sedative effect of midazolam HCl syrup is accentuated by any concomitantly administered medication which depresses the central nervous system, particularly opioids (e.g., morphine, meperidine, and fentanyl), propofol, ketamine, nitrous oxide, secobarbital and droperidol. Consequently, the dose of midazolam HCl syrup should be adjusted according to the type and amount of concomitant medications administered and the desired clinical response [see DOSAGE AND ADMINISTRATION] .
No significant adverse interactions with common premedications (such as atropine, scopolamine, glycopyrrolate, diazepam, hydroxyzine, and other muscle relaxants) or local anesthetics have been observed.
Inhibitors of CYP3A4 Isozymes: Caution is advised when midazolam is administered concomitantly with drugs that are known to inhibit the cytochrome P450 3A4 enzyme system (ie, some drugs in the drug classes of azole antimycotics, protease inhibitors, calcium channel antagonists, and macrolide antibiotics). Drugs such as diltiazem, erythromycin, fluconazole, itraconazole, ketoconazole, saquinavir, and verapamil were shown to significantly increase the C max and AUC of orally administered midazolam. These drug interactions may result in increased and prolonged sedation due to a decrease in plasma clearance of midazolam. Although not studied, the potent cytochrome P450 3A4 inhibitors ritonavir and nelfinavir may cause intense and prolonged sedation and respiratory depression due to a decrease in plasma clearance of midazolam. Caution is advised when midazolam HCl syrup is used concomitantly with these drugs. Dose adjustments should be considered and possible prolongation and intensity of effect should be anticipated [see CLINICAL PHARMACOLOGY: Pharmacokinetics: Special Population: Drug-Drug Interactions] .
Inducers of CYP3A4 Isozymes: Cytochrome P450 inducers, such as rifampin, carbamazepine, and phenytoin, induce metabolism and cause a markedly decreased C max and AUC of oral midazolam in adult studies. Although clinical studies have not been performed, phenobarbital is expected to have the same effect. Caution is advised when administering midazolam to patients receiving these medications and if necessary dose adjustments should be considered.
Drug/Laboratory Test Interactions
Midazolam has not been shown to interfere with results obtained in clinical laboratory tests.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: Midazolam maleate was administered with diet in mice and rats for 2 years at dosages of 1, 9, and 80 mg/kg/day. In female mice in the highest dose (10 times the highest oral dose of 1.0 mg/kg for a pediatric patient, on a mg/m 2 basis) group there was a marked increase in the incidence of hepatic tumors. In high-dose (19 times the pediatric dose) male rats there was a small but statistically significant increase in benign thyroid follicular cell tumors. Dosages of 9 mg/kg/day of midazolam maleate (1 to 2 times the pediatric dose) did not increase the incidence of tumors in mice or rats. The pathogenesis of induction of these tumors is not known. These tumors were found after chronic administration, whereas human use will ordinarily be single or intermittent doses.
Mutagenesis: Midazolam HCl syrup did not have mutagenic activity in Salmonella typhimurium(5 bacterial strains), Chinese hamster lung cells (V79), human lymphocytes or in the micronucleus test in mice.
Impairment of Fertility: Male rats were treated orally with 1, 4, or 16 mg/kg midazolam (0.2, 0.6, and 2.6 times the dose of 1 mg/kg based on body surface area) beginning 62 days prior to mating with female rats treated with the same doses for 14 days prior to mating to Gestation Day 13 or Lactation Day 21. There were no adverse effects on either male or female fertility noted.
Pregnancy
Pregnancy Exposure Registry: There is a pregnancy registry that monitors pregnancy outcomes in women exposed to psychiatric medications, including midazolam HCl syrup, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Psychiatric Medications at 1-866-961-2388 or visiting online at https://womensmentalhealth.org/pregnancyregistry/.
Risk Summary: Neonates born to mothers using benzodiazepines late in pregnancy have been reported to experience symptoms of sedation and/or neonatal withdrawal [seeWARNINGS: Neonatal Sedation and Withdrawal Syndrome and Clinical Considerations]. Available data from published observational studies of pregnant women exposed to benzodiazepines do not report a clear association with benzodiazepines and major birth defects (see Data) .
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations:Fetal/Neonatal Adverse Reactions: Benzodiazepines cross the placenta and may produce respiratory depression, hypotonia and sedation in neonates. Monitor neonates exposed to midazolam HCl syrup during pregnancy and labor for signs of sedation, respiratory depression, hypotonia, and feeding problems. Monitor neonates exposed to midazolam HCl syrup during pregnancy for signs of withdrawal. Manage these neonates accordingly [see WARNINGS: Neonatal Sedation and Withdrawal Syndrome] .
Data:Human Data: Published data from observational studies on the use of benzodiazepines during pregnancy do not report a clear association with benzodiazepines and major birth defects. Although early studies reported an increased risk of congenital malformations with diazepam and chlordiazepoxide, there was no consistent pattern noted. In addition, the majority of more recent case-control and cohort studies of benzodiazepine use during pregnancy, which were adjusted for confounding exposures to alcohol, tobacco and other medications, have not confirmed these findings.
Animal Data: Pregnant rats were treated with midazolam using intravenous doses of 0.2, 1, and 4 mg/kg/day (0.09, 0.46, and 1.85 times the human induction dose of 0.35 mg/kg based on body surface area comparisons) during the period of organogenesis (Gestation Day 7 through 15). Midazolam did not cause adverse effects to the fetus at doses of up to 1.85 times the human induction dose. All doses produced slight to moderate ataxia. The high dose produced a 5% decrease in maternal body weight gain compared to control.
Pregnant rabbits were treated with midazolam using intravenous doses of 0.2, 0.6, and 2 mg/kg/day (0.09, 0.46, and 1.85 times the human induction dose of 0.35 mg/kg based on body surface area comparisons) during the period of organogenesis (Gestation Day 7 to 18). Midazolam did not cause adverse effects to the fetus at doses of up to 1.85 times the human induction dose. The high dose was associated with findings of ataxia and sedation but no evidence of maternal toxicity.
Pregnant rats were administered midazolam using intravenous doses of 0.2, 1, and 4 mg/kg/day (0.09, 0.46, and 1.85 times the human induction dose of 0.35 mg/kg based on body surface area comparisons) during late gestation and through lactation (Gestation Day 15 through Lactation Day 21). All doses produced ataxia. The high dose produced a slight decrease in maternal body weight gain compared to control. There were no clear adverse effects noted in the offspring. The study included no functional assessments of the pups, such as learning and memory testing or reproductive capacity.
In a published study in primates, administration of an anesthetic dose of ketamine for 24 hours on Gestation Day 122 increased neuronal apoptosis in the developing brain of the fetus. In other published studies, administration of either isoflurane or propofol for 5 hours on Gestation Day 120 resulted in increased neuronal and oligodendrocyte apoptosis in the developing brain of the offspring. With respect to brain development, this time period corresponds to the third trimester of gestation in the human. The clinical significance of these findings is not clear; however, studies in juvenile animals suggest neuroapoptosis correlates with long-term cognitive deficits [see WARNINGS, Pediatric Neurotoxicity, PRECAUTIONS, Pediatric Use, and ANIMAL PHARMACOLOGY AND/OR TOXICOLOGY] .
Nursing Mothers
Risk Summary: There are reports of sedation, poor feeding, and poor weight gain in infants exposed to benzodiazepines through breast milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Midazolam HCl syrup and any potential adverse effects on the breastfed infants from Midazolam HCl syrup or from the underlying maternal condition.
Clinical Considerations: Infants exposed to Midazolam HCl syrup through breast milk should be monitored for sedation, poor feeding and poor weight gain. A lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk during treatment for a range of at least 4 to 8 hours after midazolam administration in order to minimize drug exposure to a breastfed infant.
Pediatric Use
Published juvenile animal studies demonstrate that the administration of anesthetic and sedation drugs, such as Midazolam Hydrochloride Syrup 2 mg/mL, that either block NMDA receptors or potentiate the activity of GABA during the period of rapid brain growth or synaptogenesis, results in widespread neuronal and oligodendrocyte cell loss in the developing brain and alterations in synaptic morphology and neurogenesis. Based on comparisons across species, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester of gestation through the first several months of life, but may extend out to approximately 3 years of age in humans.
In primates, exposure to 3 hours of ketamine that produced a light surgical plane of anesthesia did not increase neuronal cell loss, however, treatment regimens of 5 hours or longer of isoflurane increased neuronal cell loss. Data from isoflurane-treated rodents and ketamine-treated primates suggest that the neuronal and oligodendrocyte cell losses are associated with prolonged cognitive deficits in learning and memory. The clinical significance of these nonclinical findings is not known, and healthcare providers should balance the benefits of appropriate anesthesia in pregnant women, neonates, and young children who require procedures with the potential risks suggested by the nonclinical data. [see WARNINGS; Pediatric Neurotoxicity, PRECAUTIONS; Pregnancy, and Pediatric Use and ANIMAL PHARMACOLOGY AND/OR TOXICOLOGY] .
Geriatric Use
The safety and efficacy of this product have not been fully studied in geriatric patients. Therefore, there are no available data on a safe dosing regimen. One study in geriatric subjects, using midazolam 7.5 mg as a premedicant prior to general anesthesia, noted a 60% incidence of hypoxemia (pO 2 <90% for over 30 seconds) at sometime during the operative procedure versus 15% for the nonpremedicated group. Until further information is available it is recommended that this product should not be used in geriatric patients.
Use in Patients With Heart Disease
Following oral administration of 7.5 mg of midazolam to adult patients with congestive heart failure, the half-life of midazolam was 43% higher than in control subjects. One study suggests that hypercarbia or hypoxia following premedication with oral midazolam might pose a risk to children with congenital heart disease and pulmonary hypertension, although there are no known reports of pulmonary hypertensive crisis that had been triggered by premedication. In the study, 22 children were premedicated with oral midazolam (0.75 mg/kg) or IM morphine plus scopolamine prior to elective repair of congenital cardiac defects. Both premedication regimens increased PtcCO 2 and decreased SpO 2 and respiratory rates preferentially in patients with pulmonary hypertension.
ADVERSE REACTIONS
The distribution of adverse events occurring in patients evaluated in a randomized, double-blind, parallel-group trial are presented in Tables 5 and 6 by body system in order of decreasing frequency: for the premedication period (eg, sedation period prior to induction of anesthesia) alone, see Table 5; for over the entire monitoring period including premedication, anesthesia and recovery, see Table 6.
The distribution of adverse events occurring during the premedication period, before induction of anesthesia, is presented in Table 5. Emesis, which occurred in 31/397 (8%) patients over the entire monitoring period, occurred in 3/397 (0.8%) of patients during the premedication period (from midazolam administration to mask induction). Nausea, which occurred in 14/397 (4%) patients over the entire monitoring period (premedication, anesthesia and recovery), occurred in 2/397 (0.5%) patients during the premedication period.
This distribution of all adverse events occurring in ≥1% of patients over the entire monitoring period are presented in Table 6. For the entire monitoring period (premedication, anesthesia and recovery), adverse events were reported by 82/397 (21%) patients who received midazolam overall. The most frequently reported adverse events were emesis occurring in 31/397 (8%) patients and nausea occurring in 14/397 (4%) patients. Most of these gastrointestinal events occurred after the administration of other anesthetic agents.
For the respiratory system overall, adverse events (hypoxia, laryngospasm, rhonchi, coughing, respiratory depression, airway obstruction, upper-airway congestion, shallow respirations), occurred during the entire monitoring period in 31/397 (8%) patients and increased in frequency as dosage was increased: 7/132 (5%) patients in the 0.25 mg/kg dose group, 9/132 (7%) patients in the 0.5 mg/kg dose group, and 15/133 (11%) patients in the 1.0 mg/kg dose group.
Most of the respiratory adverse events occurred during induction, general anesthesia or recovery. One patient (0.25%) experienced a respiratory system adverse event (laryngospasm) during the premedication period. This adverse event occurred precisely at the time of induction. Although many of the respiratory complications occurred in settings of upper airway procedures or concurrently administered opioids, a number of these events occurred outside of these settings as well. In this study, administration of midazolam HCl syrup was generally accompanied by a slight decrease in both systolic and diastolic blood pressures, as well as a slight increase in heart rate.
|
||||||||
|
Body System |
Treatment Regimen |
Overall |
||||||
|
No. Patients with Adverse Events |
0.25 mg/kg (n=132) |
0.50 mg/kg (n=132) |
1.0 mg/kg (n=133) |
(n=397) |
||||
|
No. |
(%) |
No. |
(%) |
No. |
(%) |
No. |
(%) |
|
|
Gastrointestinal System Disorders |
||||||||
|
Emesis |
1 |
(0.76%) |
1 |
(0.76%) |
1 |
(0.75%) |
3 |
(0.76%) |
|
Nausea |
2 |
(1.5%) |
2 |
(0.50%) |
||||
|
Respiratory System Disorders |
||||||||
|
Laryngospasm |
1 * |
(0.75%) |
1 |
(0.25%) |
||||
|
Sneezing/Rhinorrhea |
1 |
(0.75%) |
1 |
(0.25%) |
||||
|
ALL BODY SYSTEMS |
1 |
(0.76%) |
1 |
(0.76%) |
5 |
(3.8%) |
7 |
(1.8%) |
|
Body System |
Treatment Regimen |
Overall |
||||||
|
No. Patients with Adverse Events |
0.25 mg/kg (n=132) |
0.50 mg/kg (n=132) |
1.0 mg/kg (n=133) |
(n=397) |
||||
|
No. |
(%) |
No. |
(%) |
No. |
(%) |
No. |
(%) |
|
|
Gastrointestinal System Disorders |
||||||||
|
Emesis |
11 |
(8%) |
5 |
(4%) |
15 |
(11%) |
31 |
(8%) |
|
Nausea |
6 |
(5%) |
2 |
(2%) |
6 |
(5%) |
14 |
(4%) |
|
Overall |
16 |
(12%) |
8 |
(6%) |
16 |
(12%) |
40 |
(10%) |
|
Respiratory System Disorders |
||||||||
|
Hypoxia |
0 |
5 |
(4%) |
4 |
(3%) |
9 |
(2%) |
|
|
Laryngospasm |
0 |
1 |
(<1%) |
5 |
(4%) |
6 |
(2%) |
|
|
Respiratory Depression |
2 |
(2%) |
1 |
(<1%) |
2 |
(2%) |
5 |
(1%) |
|
Rhonchi |
2 |
(2%) |
1 |
(<1%) |
2 |
(2%) |
5 |
(1%) |
|
Airway Obstruction |
2 |
(2%) |
2 |
(2%) |
0 |
4 |
(1%) |
|
|
Upper Airway Congestion |
2 |
(2%) |
0 |
2 |
(2%) |
4 |
(1%) |
|
|
Overall |
7 |
(5%) |
9 |
(7%) |
15 |
(11%) |
31 |
(8%) |
|
Psychiatric Disorders |
||||||||
|
Agitated |
1 |
(<1%) |
2 |
(2%) |
3 |
(2%) |
6 |
(2%) |
|
Overall |
1 |
(<1%) |
3 |
(2%) |
4 |
(3%) |
8 |
(2%) |
|
Heart Rate, Rhythm Disorders |
||||||||
|
Bradycardia |
1 |
(<1%) |
3 |
(2%) |
0 |
4 |
(1%) |
|
|
Bigeminy |
2 |
(2%) |
0 |
0 |
2 |
(<1%) |
||
|
Overall |
3 |
(2%) |
3 |
(2%) |
1 |
(<1%) |
7 |
(2%) |
|
Central & Peripheral Nervous System Disorders |
||||||||
|
Prolonged Sedation |
0 |
0 |
2 |
(2%) |
2 |
(<1%) |
||
|
Overall |
2 |
(2%) |
0 |
3 |
(2%) |
5 |
(1%) |
|
|
Skin and Appendages Disorders |
||||||||
|
Rash |
2 |
(2%) |
0 |
0 |
2 |
(<1%) |
||
|
Overall |
2 |
(2%) |
2 |
(2%) |
0 |
4 |
(1%) |
|
|
ALL BODY SYSTEMS |
26 |
(20%) |
23 |
(17%) |
33 |
(25%) |
82 |
(21%) |
There were no deaths during the study and no patient withdrew from the study due to adverse events. Serious adverse events (both respiratory disorders) were experienced postoperatively by two patients: one case of airway obstruction and desaturation (SpO 2 of 33%) in a patient given midazolam HCl syrup 0.25 mg/kg, and one case of upper airway obstruction and respiratory depression following 0.5 mg/kg. Both patients had received intravenous morphine sulfate (1.5 mg total for both patients).
Other adverse events that have been reported in the literature with the oral administration of midazolam (not necessarily midazolam HCl syrup), are listed below. The incidence rate for these events was generally <1%.
Respiratory: apnea, hypercarbia, desaturation, stridor.
Cardiovascular: decreased systolic and diastolic blood pressure, increased heart rate.
Gastrointestinal: nausea, vomiting, hiccoughs, gagging, salivation, drooling.
Central Nervous System: dysphoria, disinhibition, excitation, aggression, mood swings, hallucinations, adverse behavior, agitation, dizziness, confusion, ataxia, vertigo, dysarthria.
Special Senses: diplopia, strabismus, loss of balance, blurred vision.
DRUG ABUSE AND DEPENDENCE
Midazolam HCl syrup is a benzodiazepine and is a Schedule IV controlled substance that can produce drug dependence of the diazepam-type. Therefore, midazolam HCl syrup may be subject to misuse, abuse and addiction. Benzodiazepines can cause physical dependence. Physical dependence results in withdrawal symptoms in patients who abruptly discontinue the drug. Withdrawal symptoms (ie, convulsions, hallucinations, tremors, abdominal and muscle cramps, vomiting and sweating), similar in characteristics to those noted with barbiturates and alcohol have occurred following abrupt discontinuation of midazolam following chronic administration. Abdominal distention, nausea, vomiting, and tachycardia are prominent symptoms of withdrawal in infants.
The handling of midazolam HCl syrup should be managed to minimize the risk of diversion, including restriction of access and accounting procedures as appropriate to the clinical setting and as required by law.
OVERDOSAGE
Clinical Presentation
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion, dysarthria, lethargy, hypnotic state, diminished reflexes, ataxia, and hypotonia. Rarely, paradoxical or disinhibitory reactions (including agitation, irritability, impulsivity, violent behavior, confusion, restlessness, excitement, and talkativeness) may occur. In severe overdosage cases, patients may develop respiratory depression and coma. Overdosage of benzodiazepines in combination with other CNS depressants (including alcohol and opioids) may be fatal [see WARNINGS: Dependence and Withdrawal Reactions] . Markedly abnormal (lowered or elevated) blood pressure, heart rate, or respiratory rate raise the concern that additional drugs and/or alcohol are involved in the overdosage.
Management of Overdose
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway management. Flumazenil, a specific benzodiazepine receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines in the management of benzodiazepine overdosage, can lead to withdrawal and adverse reactions, including seizures, particularly in the context of mixed overdosage with drugs that increase seizure risk (e.g., tricyclic and tetracyclic antidepressants) and in patients with long term benzodiazepine use and physical dependency. The risk of withdrawal seizures with flumazenil use may be increased in patients with epilepsy. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of a potentially life-threatening condition (e.g., status epilepticus). If the decision is made to use flumazenil, it should be used as an adjunct to, not as a substitute for, supportive management of benzodiazepine overdosage. See the flumazenil injection Prescribing Information.
Consider contacting a poison center (1-800-222-1222), poisoncontrol.org, or a medical toxicologist for additional overdosage management recommendations.
DOSAGE AND ADMINISTRATION
Midazolam HCl syrup is indicated for use as a single dose (0.25 to 1.0 mg/kg with a maximum dose of 20 mg) for preprocedural sedation and anxiolysis in pediatric patients. Midazolam HCl syrup is not intended for chronic administration.
Monitoring
Midazolam HCl syrup should only be used in hospital or ambulatory care settings, including physicians’ and dentists’ offices that can provide for continuous monitoring of respiratory and cardiac function. Immediate availability of resuscitative drugs and age- and size-appropriate equipment for bag/valve/mask ventilation and intubation, and personnel trained in their use and skilled in airway management should be assured [see WARNINGS] . For deeply sedated patients, a dedicated individual whose sole responsibility it is to observe the patient, other than the practitioner performing the procedure, should monitor the patient throughout the procedure. Continuous monitoring of respiratory and cardiac function is required.
Midazolam HCl syrup must be given only to patients if they will be monitored by direct visual observation by a health care professional. Midazolam HCl syrup should only be administered by persons specifically trained in the use of anesthetic drugs and the management of respiratory effects of anesthetic drugs, including respiratory and cardiac resuscitation of patients in the age group being treated.
Patient response to sedative agents, and resultant respiratory status, is variable. Regardless of the intended level of sedation or route of administration, sedation is a continuum; a patient may move easily from light to deep sedation, with potential loss of protective reflexes, particularly when coadministered with anesthetic agents, other CNS depressants, and concomitant medications which may potentially cause a more intense and prolonged sedation [seePRECAUTIONS: Drug Interactions]. This is especially true in pediatric patients. The health care practitioner who uses this medication in pediatric patients should be aware of and follow accepted professional guidelines for pediatric sedation appropriate to their situation.
Sedation guidelines recommend a careful presedation history to determine how a patient’s underlying medical conditions or concomitant medications might affect their response to sedation/analgesia as well as a physical examination including a focused examination of the airway for abnormalities. Further recommendations include appropriate presedation fasting.
Intravenous access is not thought to be necessary for all pediatric patients sedated for a diagnostic or therapeutic procedure because in some cases the difficulty of gaining IV access would defeat the purpose of sedating the child; rather, emphasis should be placed upon having the intravenous equipment available and a practitioner skilled in establishing vascular access in pediatric patients immediately available.
Midazolam HCl syrup must never be used without individualization of dosage, particularly when used with other medications capable of producing CNS depression. Younger (<6 years of age) pediatric patients may require higher dosages (mg/kg) than older pediatric patients, and may require close monitoring.
When midazolam HCl syrup is given in conjunction with opioids or other sedatives, the potential for respiratory depression, airway obstruction, or hypoventilation is increased. For appropriate patient monitoring, see WARNINGS and DOSAGE AND ADMINISTRATION: Monitoring. The health care practitioner who uses this medication in pediatric patients should be aware of and follow accepted professional guidelines for pediatric sedation appropriate to their situation.
The recommended dose for pediatric patients is a single dose of 0.25 to 0.5 mg/kg, depending on the status of the patient and desired effect, up to a maximum dose of 20 mg. In general, it is recommended that the dose be individualized and modified based on patient age, level of anxiety, concomitant medications, and medical need [see WARNINGS and PRECAUTIONS] . The younger (6 months to <6 years of age) and less cooperative patients may require a higher than usual dose up to 1.0 mg/kg. A dose of 0.25 mg/kg may suffice for older (6 to <16 years of age) or cooperative patients, especially if the anticipated intensity and duration of sedation is less critical. For all pediatric patients, a dose of 0.25 mg/kg should be considered when midazolam HCl syrup is administered to patients with cardiac or respiratory compromise, other higher risk surgical patients, and patients who have received concomitant narcotics or other CNS depressants. As with any potential respiratory depressant, these patients must be monitored for signs of cardiorespiratory depression after receiving midazolam HCl syrup. In obese pediatric patients, the dose should be calculated based on ideal body weight. Midazolam HCl syrup has not been studied, nor is it intended for chronic use.
DISPOSAL OF MIDAZOLAM HCl SYRUP
The disposal of Schedule IV controlled substances must be consistent with State and Federal Regulations.
HOW SUPPLIED
Midazolam Hydrochloride Syrup
Midazolam HCl Syrup is supplied as a clear, red to purplish-red, cherry-brandy flavored syrup containing midazolam hydrochloride equivalent to 2 mg of midazolam/mL; and supplied in the following delivery volumes:
2.5 mL unit dose cups (5 mg per 2.5 mL)
30 cups (3 × 10) NDC 60687-576-10
5 mL unit dose cups (10 mg per 5 mL)
30 cups (3 × 10) NDC 60687-576-86
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
DO NOT USE IF SEAL IS BROKEN.
ANIMAL PHARMACOLOGY AND/OR TOXICOLOGY
Published studies in animals demonstrate that the use of anesthetic agents during the period of rapid brain growth or synaptogenesis results in widespread neuronal and oligodendrocyte cell loss in the developing brain and alterations in synaptic morphology and neurogenesis. Based on comparisons across species, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester through the first several months of life, but may extend out to approximately 3 years of age in humans.
In primates, exposure to 3 hours of exposure to an anesthetic regimen that produced a light surgical plane of anesthesia did not increase neuronal cell loss, however, treatment regimens of 5 hours or longer increased neuronal cell loss. Data in rodents and in primates suggest that the neuronal and oligodendrocyte cell losses are associated with subtle but prolonged cognitive deficits in learning and memory. The clinical significance of these nonclinical findings is not known, and healthcare providers should balance the benefits of appropriate anesthesia in neonates and young children who require procedures against the potential risks suggested by the nonclinical data [see WARNINGS; Pediatric Neurotoxicity and PRECAUTIONS; Pregnancy and Pediatric Use] .
PACKAGING INFORMATION
American Health Packaging unit dose cups (see
How Supplied section) contain drug product from Hikma Pharmaceuticals USA Inc. as follows:
(5 mg per 2.5 mL / 30 UD) NDC 60687-576-10 packaged from NDC 0054-3566
(10 mg per 5 mL / 30 UD) NDC 60687-576-86 packaged from NDC 0054-3566
Distributed By:
American Health Packaging
Columbus, OH 43217
8457610/0323F
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL- Label

Midazolam
Hydrochloride CIV
Syrup
Rx Only
FOR INSTITUTIONAL USE ONLY
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room
Temperature.]
8457610/0323F
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - Cup - 5 mg/2.5mL

Rx Only
NDC 60687- 576-03
Midazolam
Hydrochloride CIV
Syrup
5 mg/2.5 mL
Cherry-Brandy Flavor
Delivers 2.5 mL
See package insert for full
prescribing information and storage.
For Institutional Use Only.
American Health Packaging
Columbus, OH 43217
0457610/0920
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - Cup - 10mg/5mL

Rx Only
NDC 60687- 576-40
Midazolam
Hydrochloride CIV
Syrup
10 mg/5 mL
Cherry-Brandy Flavor
Delivers 5 mL
See package insert for full
prescribing information and storage.
For Institutional Use Only.
American Health Packaging
Columbus, OH 43217
0457686/0920