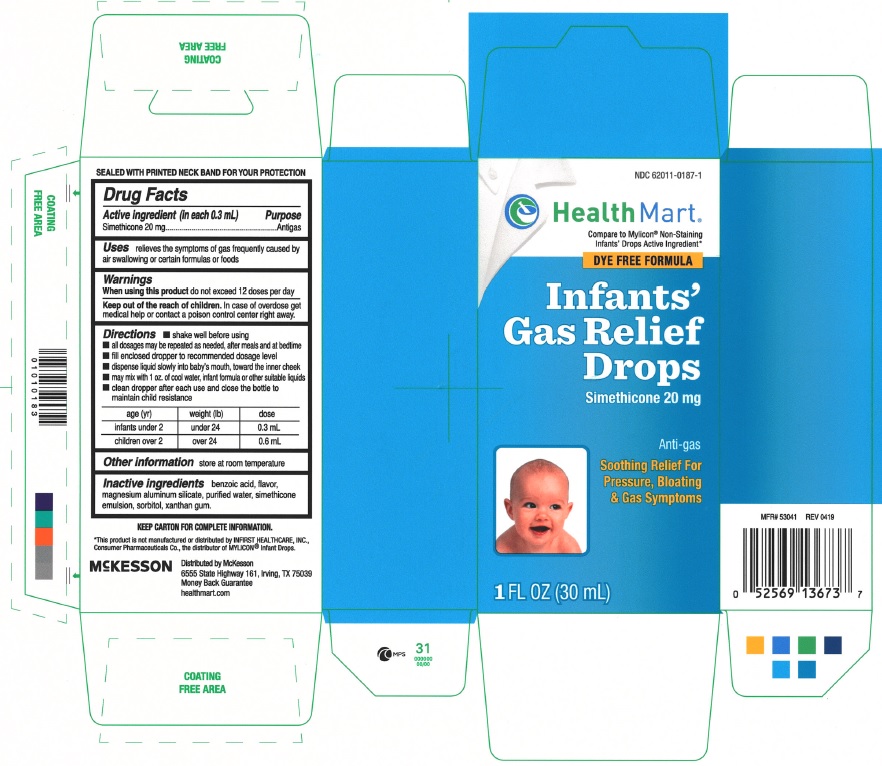
INFANTS GAS RELIEF DROPS
- simethicone emulsion
Strategic Sourcing Services LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (in each 0.3 mL)
simethicone 20 mg
Uses
relieves the symptoms of gas frequently caused by air swallowing or certain formulas or foods
Warnings
When using this product
do not exceed 12 doses per day
Keep out of reach of children. In case of overdose get medical help or contact a poison control center right away.
Directions
- shake well before using
- all dosages may be repeated as needed, after meals and at bedtime
- fill enclosed dropper to recommend dosage level
- dispense liquid slowly into baby's mouth, toward the inner cheek
- may mix with 1 oz. of cool water, infant formula or other suitable liquids
- clean dropper after each use and close the bottle to maintain child resistance
| age (yr) | weight (lb) | dose |
| infants under2 | under 24 | 0.3 mL |
| children over 2 | over 24 | 0.6 mL |
Other information
store at room temperature
Inactive ingredients
benzoic acid, flavor, magnesium aluminum silicate, purified water, simethicone emulsion, sorbitol, xanthan gum
Principal Display Panel
NDC 62011-0187-1
Compare to Mylicon® Non-Staining Infants' Drops Active Ingredient*
DYE FREE FORMULA
Infants' Gas Relief Drops
Simethicone 20 mg
Anti-gas
Soothing Relief For Pressure, Bloating & Gas Symptoms
1 fl oz (30 mL)