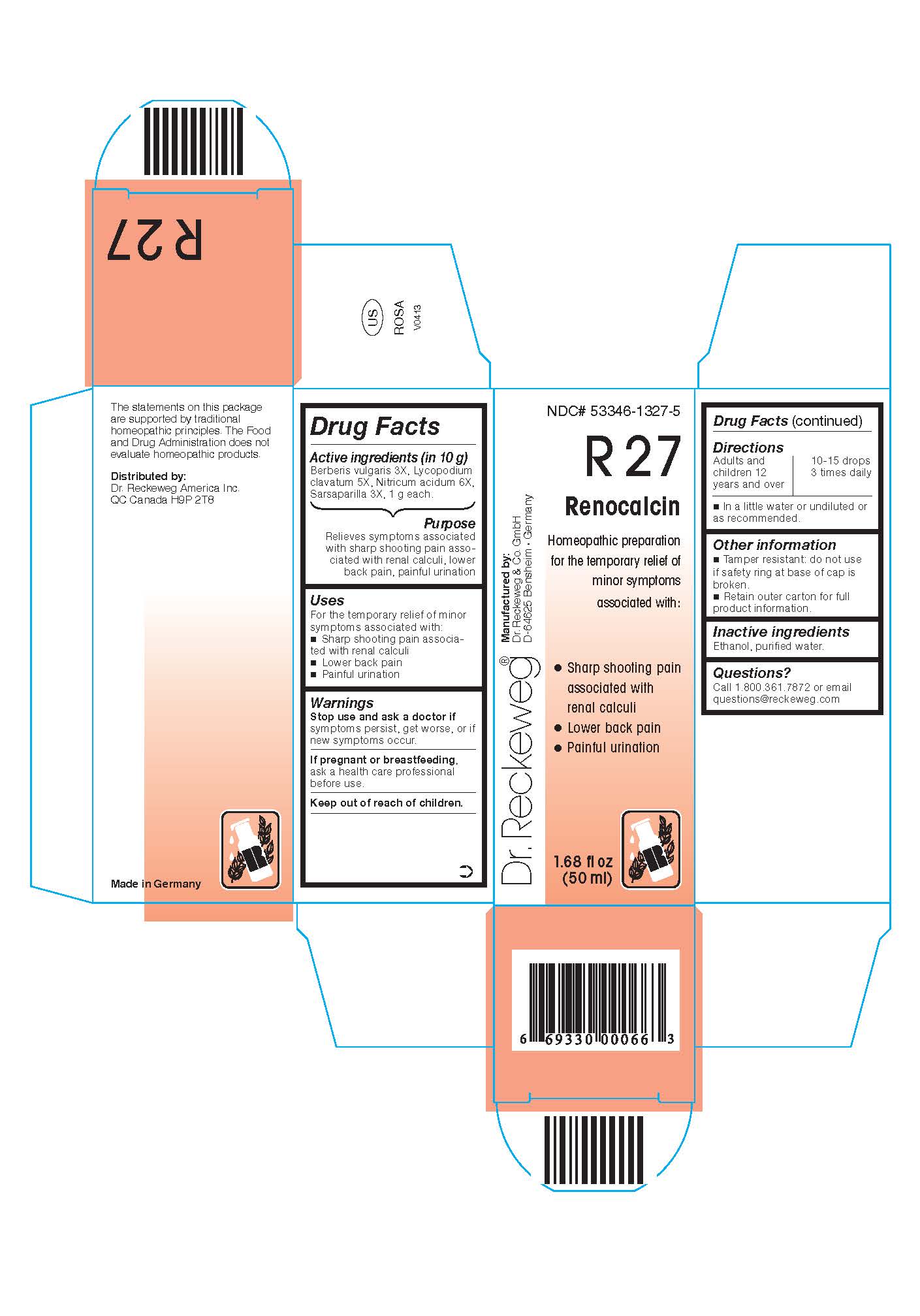
DR. RECKEWEG R27 RENOCALCIN COMBINATION PRODUCT- berberis vulgaris 3x, lycopodium clavatum 5x, nitricum acidum 6x, sarsaparilla 3x liquid
PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active ingredients:
Berberis vulgaris 3X, Lycopodium clavatum 5X, Nitricum acidum 6X, Sarsaparilla 3X, 1 g each in 10 g.
Purpose
Relieves symptoms associated with sharp shooting pain associated with renal calculi, lower back pains, painful urination
Uses
For the temporary relief of minor symptoms associated with:
- Sharp shooting pain associated with renal calculi
- Lower back pains
- Painful urination
Warnings
Stop use and ask a doctor if symptoms persist, get worse, or if new symptoms occur.
If pregnant or breastfeeding, ask a health care professional before use.
Keep out of reach of children.
Directions
Adults and children ≥ 12 years 10-15 drops 3 times daily in a little water or undiluted or as recommended.
Other information
Tamper resistant: do not use if safety ring at base of cap is broken.
Retain outer carton for full product instructions.
Inactive ingredients
Ethanol, purified water.
Questions?
Call 1-800-361-7872 or email questions@reckeweg.com
NDC# 53346-1327-5
Dr. Reckeweg R27 Renocalcin
Homeopathic preparation for the temporary relief of minor symptoms associated with:
- Sharp shooting pain associated with renal calculi
- Lower back pains
- Painful urination
Manufactured by:
Dr. Reckeweg Co. GmbH
D-64625 Bensheim
Germany
1.68 fl oz
(50 ml)
PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO
