FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Primary Hyperlipidemia
WELCHOL is indicated as an adjunct to diet and exercise to reduce elevated low-density lipoprotein cholesterol (LDL-C) in adults with primary hyperlipidemia (Fredrickson Type IIa) as monotherapy or in combination with an hydroxymethyl-glutaryl-coenzyme A (HMG CoA) reductase inhibitor (statin).
WELCHOL is indicated as monotherapy or in combination with a statin to reduce LDL-C levels in boys and postmenarchal girls, 10 to 17 years of age, with heterozygous familial hypercholesterolemia if after an adequate trial of diet therapy the following findings are present:
a. LDL-C remains ≥ 190 mg/dL or
b. LDL-C remains ≥ 160 mg/dL and
- there is a positive family history of premature cardiovascular disease or
- two or more other CVD risk factors are present in the pediatric patient.
Lipid-altering agents should be used in addition to a diet restricted in saturated fat and cholesterol when response to diet and non-pharmacological interventions alone has been inadequate [See Clinical Studies (14.1)].
In patients with coronary heart disease (CHD) or CHD risk equivalents such as diabetes mellitus, LDL-C treatment goals are < 100 mg/dL. An LDL-C goal of < 70 mg/dL is a therapeutic option on the basis of recent trial evidence. If LDL-C is at goal but the serum triglyceride (TG) value is > 200 mg/dL, then non-HDL cholesterol (non-HDL-C) (total cholesterol [TC] minus high density lipoprotein cholesterol [HDL-C]) becomes a secondary target of therapy. The goal for non-HDL-C in persons with high serum TG is set at 30 mg/dL higher than that for LDL-C.
1.2 Type 2 Diabetes Mellitus
WELCHOL is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus [See Clinical Studies (14.2)].
Diabetes mellitus is considered a CHD risk equivalent. In addition to glycemic control, intensive lipid control is warranted [See Indications and Usage (1.1) and Warnings and Precautions (5.2)].
1.3 Important Limitations of Use
- WELCHOL should not be used for the treatment of type 1 diabetes or for the treatment of diabetic ketoacidosis.
- WELCHOL has not been studied in type 2 diabetes as monotherapy or in combination with a dipeptidyl peptidase 4 inhibitor and has not been extensively studied in combination with thiazolidinediones.
- WELCHOL has not been studied in Fredrickson Type I, III, IV, and V dyslipidemias.
- WELCHOL has not been studied in children younger than 10 years of age or in pre-menarchal girls.
2 DOSAGE AND ADMINISTRATION
2.1 Primary Hyperlipidemia
The recommended dose of WELCHOL Tablets in adults, whether used as monotherapy or in combination with a statin, is 6 tablets once daily or 3 tablets twice daily. WELCHOL Tablets should be taken with a meal and liquid.
The recommended dose of WELCHOL for Oral Suspension, in adults and children 10 to 17 years of age, is one 3.75 gram packet once daily or one 1.875 gram packet twice daily. To prepare, empty the entire contents of one packet into a glass or cup. Add ½ to 1 cup (4 to 8 ounces) of water. Stir well and drink. WELCHOL for Oral Suspension should be taken with meals. To avoid esophageal distress, WELCHOL for Oral Suspension should not be taken in its dry form. Due to tablet size, it is recommended that any patient who has difficulty swallowing tablets use WELCHOL for Oral Suspension.
WELCHOL can be dosed at the same time as a statin or the two drugs can be dosed apart [See Clinical Studies (14.1)].
After initiation of WELCHOL, lipid levels should be analyzed within 4 to 6 weeks.
2.2 Type 2 Diabetes Mellitus
The recommended dose of WELCHOL Tablets is 6 tablets once daily or 3 tablets twice daily. WELCHOL should be taken with a meal and liquid.
The recommended dose of WELCHOL for Oral Suspension is one 3.75 gram packet once daily or one 1.875 gram packet twice daily. To prepare, empty the entire contents of one packet into a glass or cup. Add ½ to 1 cup (4 to 8 ounces) of water. Stir well and drink. WELCHOL for Oral Suspension should be taken with meals. To avoid esophageal distress, WELCHOL for Oral Suspension should not be taken in its dry form.
3 DOSAGE FORMS AND STRENGTHS
- Tablets: 625 mg tablets are off-white, oval, film-coated and imprinted with "Sankyo" and "C01" on one side.
- Oral Suspension: a white to pale yellow powder containing yellow granules packaged in single-dose packets: 3.75 gram single-dose packet, 1.875 gram single-dose packet.
5 WARNINGS AND PRECAUTIONS
5.1 General
The effect of WELCHOL on cardiovascular morbidity and mortality has not been determined.
5.2 Serum Triglycerides (TG)
WELCHOL, like other bile acid sequestrants, can increase serum TG concentrations.
WELCHOL had small effects on serum TG (median increase 5% compared to placebo) in trials of patients with primary hyperlipidemia [See Adverse Reactions (6.1) and Clinical Studies (14.1)].
In clinical trials in patients with type 2 diabetes, greater increases in TG levels occurred when WELCHOL was used in combination with sulfonylureas (median increase 18% compared to placebo in combination with sulfonylureas) and when WELCHOL was used in combination with insulin (median increase 22% compared to placebo in combination with insulin) [See Adverse Reactions (6.1) and Clinical Studies (14.2)]. Hypertriglyceridemia of sufficient severity can cause acute pancreatitis. The long-term effect of hypertriglyceridemia on the risk of coronary artery disease is uncertain. In patients with type 2 diabetes, the effect of WELCHOL on LDL-C levels may be attenuated by WELCHOL’s effects on TG levels and a smaller reduction in non-HDL-C compared to the reduction in LDL-C. Caution should be exercised when treating patients with TG levels greater than 300 mg/dL. Because most patients in the WELCHOL clinical trials had baseline TG <300 mg/dL, it is unknown whether patients with more uncontrolled baseline hypertriglyceridemia would have greater increases in serum TG levels with WELCHOL. In addition, the use of WELCHOL is contraindicated in patients with TG levels >500 mg/dL [See Contraindications (4)]. Lipid parameters, including TG levels and non-HDL-C, should be obtained before starting WELCHOL and periodically thereafter. WELCHOL should be discontinued if TG levels exceed 500 mg/dL or if the patient develops hypertriglyceridemia-induced pancreatitis [See Adverse Reactions (6.1)].
5.3 Vitamin K or Fat-Soluble Vitamin Deficiencies Precautions
Bile acid sequestrants may decrease the absorption of fat-soluble vitamins A, D, E, and K. No specific clinical studies have been conducted to evaluate the effects of WELCHOL on the absorption of co-administered dietary or supplemental vitamin therapy. In non-clinical safety studies, rats administered colesevelam hydrochloride at doses greater than 30-fold the projected human clinical dose experienced hemorrhage from vitamin K deficiency. Patients on oral vitamin supplementation should take their vitamins at least 4 hours prior to WELCHOL. Caution should be exercised when treating patients with a susceptibility to deficiencies of vitamin K (e.g., patients on warfarin, patients with malabsorption syndromes) or other fat-soluble vitamins.
5.4 Gastrointestinal Disorders
Because of its constipating effects, WELCHOL is not recommended in patients with gastroparesis, other gastrointestinal motility disorders, and in those who have had major gastrointestinal tract surgery and who may be at risk for bowel obstruction. Because of the tablet size, WELCHOL Tablets can cause dysphagia or esophageal obstruction and should be used with caution in patients with dysphagia or swallowing disorders. To avoid esophageal distress, WELCHOL for Oral Suspension should not be taken in its dry form. Always mix WELCHOL for Oral Suspension with water before ingesting.
5.5 Drug Interactions
WELCHOL reduces gastrointestinal absorption of some drugs. Drugs with a known interaction with colesevelam should be administered at least 4 hours prior to WELCHOL. Drugs that have not been tested for interaction with colesevelam, especially those with a narrow therapeutic index, should also be administered at least 4 hours prior to WELCHOL. Alternatively, the physician should monitor drug levels of the co-administered drug [See Drug Interactions (7) and Clinical Pharmacology (12.3)].
5.6 Phenylketonurics
WELCHOL for Oral Suspension contains 24 mg phenylalanine per 1.875 gram packet and 48 mg phenylalanine per 3.75 gram packet [See Description (11)].
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in clinical studies of another drug and may not reflect the rates observed in practice.
In the lipid-lowering trials, 807 adult patients received at least one dose of WELCHOL (total exposure 199 patient-years). In the type 2 diabetes trials, 566 patients received at least one dose of WELCHOL (total exposure 209 patient-years).
In clinical trials for the reduction of LDL-C, 68% of patients receiving WELCHOL vs. 64% of patients receiving placebo reported an adverse reaction. In clinical trials of type 2 diabetes, 60% of patients receiving WELCHOL vs. 56% of patients receiving placebo reported an adverse reaction.
Primary Hyperlipidemia: In 7 double-blind, placebo-controlled, clinical trials, 807 patients with primary hyperlipidemia (age range 18-86 years, 50% women, 90% Caucasians, 7% Blacks, 2% Hispanics, 1% Asians) and elevated LDL-C were treated with WELCHOL 1.5 g/day to 4.5 g/day from 4 to 24 weeks.
| Number of Patients (%) | ||
| WELCHOL N = 807 | Placebo N = 258 |
|
| Constipation | 89 (11.0) | 18 (7.0) |
| Dyspepsia | 67 (8.3) | 9 (3.5) |
| Nausea | 34 (4.2) | 10 (3.9) |
| Accidental injury | 30 (3.7) | 7 (2.7) |
| Asthenia | 29 (3.6) | 5 (1.9) |
| Pharyngitis | 26 (3.2) | 5 (1.9) |
| Flu syndrome | 26 (3.2) | 8 (3.1) |
| Rhinitis | 26 (3.2) | 8 (3.1) |
| Myalgia | 17 (2.1) | 1 (0.4) |
Pediatric Patients 10 to 17 Years of Age: In an 8-week double-blind, placebo-controlled study boys and post menarchal girls, 10 to 17 years of age, with heterozygous familial hypercholesterolemia (heFH) (n=192), were treated with WELCHOL tablets (1.9-3.8 g, daily) or placebo tablets [See Clinical Studies (14.1)].
| Number of Patients (%) | ||
| WELCHOL | Placebo | |
| N = 129 | N = 65 | |
| Nasopharyngitis | 8 (6.2) | 3 (4.6) |
| Headache | 5 (3.9) | 2 (3.1) |
| Fatigue | 5 (3.9) | 1 (1.5) |
| Creatine Phosphokinase Increase | 3 (2.3) | 0 (0.0) |
| Rhinitis | 3 (2.3) | 0 (0.0) |
| Vomiting | 3 (2.3) | 1 (1.5) |
The reported adverse reactions during the additional 18-week open-label treatment period with WELCHOL 3.8 g per day were similar to those during the double-blind period and included headache (7.6%), nasopharyngitis (5.4%), upper respiratory tract infection (4.9%), influenza (3.8%), and nausea (3.8%) [See Clinical Studies (14.1)].
Type 2 Diabetes Mellitus: The safety of WELCHOL in patients with type 2 diabetes mellitus was evaluated in 4 double-blind, 12-26 week, placebo-controlled clinical trials. These trials involved 1128 patients (566 patients on WELCHOL; 562 patients on placebo) with inadequate glycemic control on metformin, sulfonylurea, or insulin when these agents were used alone or in combination with other anti-diabetic agents. Upon completion of the pivotal trials, 492 patients entered a 52-week open-label uncontrolled extension study during which all patients received WELCHOL 3.8 g/day while continuing background treatment with metformin, sulfonylurea, or insulin alone or in combination with other anti-diabetic agents.
A total of 6.7% of WELCHOL-treated patients and 3.2% of placebo-treated patients were discontinued from the diabetes trials due to adverse reactions. This difference was driven mostly by gastrointestinal adverse reactions such as abdominal pain and constipation.
One patient in the pivotal trials discontinued due to body rash and mouth blistering that occurred after the first dose of WELCHOL, which may represent a hypersensitivity reaction to WELCHOL.
| Number of Patients (%) | ||
| WELCHOL N = 566 | Placebo N = 562 |
|
| Constipation | 49 (8.7) | 11 (2.0) |
| Nasopharyngitis | 23 (4.1) | 20 (3.6) |
| Dyspepsia | 22 (3.9) | 8 (1.4) |
| Hypoglycemia | 17 (3.0) | 13 (2.3) |
| Nausea | 17 (3.0) | 8 (1.4) |
| Hypertension | 16 (2.8) | 9 (1.6) |
Hypertriglyceridemia: Patients with fasting serum TG levels above 500 mg/dL were excluded from the diabetes clinical trials. In the phase 3 diabetes trials, 637 (63%) patients had baseline fasting serum TG levels less than 200 mg/dL, 261 (25%) had baseline fasting serum TG levels between 200 and 300 mg/dL, 111 (11%) had baseline fasting serum TG levels between 300 and 500 mg/dL, and 9 (1%) had fasting serum TG levels greater than or equal to 500 mg/dL. The median baseline fasting TG concentration for the study population was 172 mg/dL; the median post-treatment fasting TG was 195 mg/dL in the WELCHOL group and 177 mg/dL in the placebo group. WELCHOL therapy resulted in a median placebo-corrected increase in serum TG of 5% (p=0.22), 22% (p<0.001), and 18% (p<0.001) when added to metformin, insulin and sulfonylureas, respectively [See Warnings and Precautions (5.2) and Clinical Studies (14.2)]. In comparison, WELCHOL resulted in a median increase in serum TG of 5% compared to placebo (p=0.42) in a 24-week monotherapy lipid-lowering trial [See Clinical Studies (14.1)].
Treatment-emergent fasting TG concentrations ≥500 mg/dL occurred in 4.1% of WELCHOL-treated patients compared to 2.0% of placebo-treated patients. Among these patients, the TG concentrations with WELCHOL (median 604 mg/dL; interquartile range 538-712 mg/dL) were similar to that observed with placebo (median 644 mg/dL; interquartile range 574-724 mg/dL). Two (0.4%) patients on WELCHOL and 2 (0.4%) patients on placebo developed TG elevations ≥1000 mg/dL. In all WELCHOL clinical trials, including studies in patients with type 2 diabetes and patients with primary hyperlipidemia, there were no reported cases of acute pancreatitis associated with hypertriglyceridemia. It is unknown whether patients with more uncontrolled, baseline hypertriglyceridemia would have greater increases in serum TG levels with WELCHOL [See Contraindications (4) and Warnings and Precautions (5.2)].
Cardiovascular adverse events: During the diabetes clinical trials, the incidence of patients with treatment-emergent serious adverse events involving the cardiovascular system was 3% (17/566) in the WELCHOL group and 2% (10/562) in the placebo group. These overall rates included disparate events (e.g., myocardial infarction, aortic stenosis, and bradycardia); therefore, the significance of this imbalance is unknown.
Hypoglycemia: Adverse events of hypoglycemia were reported based on the clinical judgment of the blinded investigators and did not require confirmation with fingerstick glucose testing. The overall reported incidence of hypoglycemia was 3.0% in patients treated with WELCHOL and 2.3% in patients treated with placebo. No WELCHOL treated patients developed severe hypoglycemia.
6.2 Post-marketing Experience
The following additional adverse reactions have been identified during post-approval use of WELCHOL. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Drug Interactions with concomitant WELCHOL administration include:
- Increased seizure activity or decreased phenytoin levels in patients receiving phenytoin. Phenytoin should be administered 4 hours prior to WELCHOL.
- Reduced International Normalized Ratio (INR) in patients receiving warfarin therapy. In warfarin-treated patients, INR should be monitored frequently during WELCHOL initiation then periodically thereafter.
- Elevated thyroid-stimulating hormone (TSH) in patients receiving thyroid hormone replacement therapy. Thyroid hormone replacement should be administered 4 hours prior to WELCHOL [See Drug Interactions (7)].
Gastrointestinal Adverse Reactions
Bowel obstruction (in patients with a history of bowel obstruction or resection), dysphagia or esophageal obstruction (occasionally requiring medical intervention), fecal impaction, pancreatitis, abdominal distension, exacerbation of hemorrhoids, and increased transaminases.
7 DRUG INTERACTIONS
Table 4 lists the drugs that have been tested in in vitro binding or in vivo drug interaction studies with colesevelam and/or drugs with postmarketing reports consistent with potential drug-drug interactions. Orally administered drugs that have not been tested for interaction with colesevelam, especially those with a narrow therapeutic index, should also be administered at least 4 hours prior to WELCHOL. Alternatively, the physician should monitor drug levels of the co-administered drug.
| Table 4 Drugs Tested in In Vitro Binding or In Vivo Drug Interaction Testing or With Post-Marketing Reports | |
| a Should be administered at least 4 hours prior to WELCHOL | |
| b No significant alteration of warfarin drug levels with warfarin and WELCHOL coadministration in an in vivo study which did not evaluate warfarin pharmacodynamics (INR). [See Post-marketing Experience (6.2)] | |
| c Cyclosporine levels should be monitored and, based on theoretical grounds, cyclosporine should be administered at least 4 hours prior to WELCHOL. | |
| Drugs with a known interaction with colesevelam | Cyclosporinec, glyburidea, levothyroxinea, and oral contraceptives containing ethinyl estradiol and norethindronea |
| Drugs with postmarketing reports consistent with potential drug-drug interactions when coadministered with WELCHOL | phenytoina, warfarinb |
| Drugs that do not interact with colesevelam based on in vitro or in vivo testing | cephalexin, ciprofloxacin, digoxin, warfarinb fenofibrate, lovastatin, metformin, metoprolol, pioglitazone, quinidine, repaglinide, valproic acid, verapamil |
In an in vivo drug interaction study, WELCHOL and warfarin coadministration had no effect on warfarin drug levels. This study did not assess the effect of WELCHOL and warfarin coadministration on INR. In postmarketing reports, concomitant use of WELCHOL and warfarin has been associated with reduced INR. Therefore, in patients on warfarin therapy, the INR should be monitored before initiating WELCHOL and frequently enough during early WELCHOL therapy to ensure that no significant alteration in INR occurs. Once the INR is stable, continue to monitor the INR at intervals usually recommended for patients on warfarin. [See Post-marketing Experience (6.2)]
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B. There are no adequate and well-controlled studies of colesevelam use in pregnant women. Animal reproduction studies in rats and rabbits revealed no evidence of fetal harm. Requirements for vitamins and other nutrients are increased in pregnancy. However, the effect of colesevelam on the absorption of fat-soluble vitamins has not been studied in pregnant women. This drug should be used during pregnancy only if clearly needed.
In animal reproduction studies, colesevelam revealed no evidence of fetal harm when administered to rats and rabbits at doses 50 and 17 times the maximum human dose, respectively. Because animal reproduction studies are not always predictive of human response, this drug should be used in pregnancy only if clearly needed.
8.3 Nursing Mothers
Colesevelam hydrochloride is not expected to be excreted in human milk because colesevelam hydrochloride is not absorbed systemically from the gastrointestinal tract.
8.4 Pediatric Use
The safety and effectiveness of WELCHOL as monotherapy or in combination with a statin were evaluated in children, 10 to 17 years of age with heFH [See Clinical Studies (14.1)]. The adverse reaction profile was similar to that of patients treated with placebo. In this limited controlled study, there were no significant effects on growth, sexual maturation, fat-soluble vitamin levels or clotting factors in the adolescent boys or girls relative to placebo [See Adverse Reactions (6.1)].
Due to tablet size, WELCHOL for Oral Suspension is recommended for use in the pediatric population. Dose adjustments are not required when WELCHOL is administered to children 10 to 17 years of age.
WELCHOL has not been studied in children younger than 10 years of age or in pre-menarchal girls.
8.5 Geriatric Use
Primary Hyperlipidemia: Of the 1350 patients enrolled in the hyperlipidemia clinical studies, 349 (26%) were ≥65 years old, and 58 (4%) were ≥75 years old. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Type 2 Diabetes Mellitus: Of the 1128 patients enrolled in the four diabetes studies, 249 (22%) were ≥65 years old, and 12 (1%) were ≥75 years old. In these trials, WELCHOL 3.8 g/day or placebo was added onto background anti-diabetic therapy. No overall differences in safety or effectiveness were observed between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Hepatic Impairment
No special considerations or dosage adjustments are recommended when WELCHOL is administered to patients with hepatic impairment.
8.7 Renal Impairment
Type 2 Diabetes Mellitus: Of the 1128 patients enrolled in the four diabetes studies, 696 (62%) had mild renal insufficiency (creatinine clearance [CrCl] 50-<80 mL/min), 53 (5%) had moderate renal insufficiency (CrCl 30-<50 mL/min), and none had severe renal insufficiency (CrCl <30 mL/min), as estimated from baseline serum creatinine using the Modification of Diet in Renal Disease (MDRD) equation. No overall differences in safety or effectiveness were observed between patients with CrCl <50 mL/min (n=53) and those with a CrCl≥50 mL/min (n=1075).
10 OVERDOSAGE
Doses of WELCHOL in excess of 4.5 g/day have not been tested. Because WELCHOL is not absorbed, the risk of systemic toxicity is low. However, excessive doses of WELCHOL may cause more severe local gastrointestinal effects (e.g., constipation) than recommended doses.
11 DESCRIPTION
WELCHOL (colesevelam hydrochloride) is a non-absorbed, polymeric, lipid-lowering and glucose-lowering agent intended for oral administration. Colesevelam hydrochloride is a high-capacity bile acid-binding molecule.
Colesevelam hydrochloride is poly(allylamine hydrochloride) cross-linked with epichlorohydrin and alkylated with 1-bromodecane and (6-bromohexyl)-trimethylammonium bromide. The chemical name (IUPAC) of colesevelam hydrochloride is allylamine polymer with 1-chloro-2,3-epoxypropane, [6-(allylamino)-hexyl]trimethylammonium chloride and N-allyldecylamine, hydrochloride. The chemical structure of colesevelam hydrochloride is represented by the following formula:
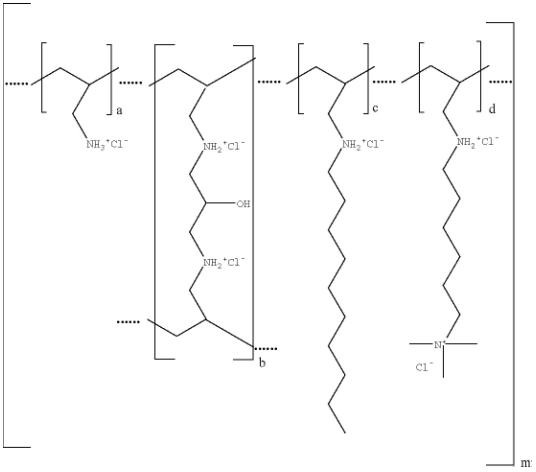
wherein (a) represents allyl amine monomer units that have not been alkylated by either of the 1-bromodecane or (6-bromohexyl)-trimethylammonium bromide alkylating agents or cross-linked by epichlorohydrin; (b) represents allyl amine units that have undergone crosslinking with epichlorohydrin; (c) represents allyl amine units that have been alkylated with a decyl group; (d) represents allyl amine units that have been alkylated with a (6-trimethylammonium) hexyl group, and m represents a number ≥ 100 to indicate an extended polymer network. A small amount of the amines are dialkylated, and are not depicted in the formula above. No regular order of the groups is implied by the structure; cross-linking and alkylation are expected to occur randomly along the polymer chains. A large amount of the amines are protonated. The polymer is depicted in the hydrochloride form; a small amount of the halides are bromide. Colesevelam hydrochloride is hydrophilic and insoluble in water.
WELCHOL Tablets are an off-white, oval, film-coated, solid tablet containing 625 mg colesevelam hydrochloride. In addition, each tablet contains the following inactive ingredients: magnesium stearate, microcrystalline cellulose, silicon dioxide, HPMC (hydroxypropyl methylcellulose), and acetylated monoglyceride. The tablets are imprinted using a water-soluble black ink.
WELCHOL for Oral Suspension is a citrus-flavored, white to pale yellow powder containing yellow granules packaged in single-dose packets containing either 1.875 gram or 3.75 gram colesevelam hydrochloride. In addition, each packet contains the following inactive ingredients: lemon flavor, orange flavor, propylene glycol alginate, simethicone, aspartame, citric acid, medium chain triglycerides, and magnesium trisilicate.
PHENYLKETONURICS: WELCHOL for Oral Suspension contains 24 mg phenylalanine per 1.875 gram dose and 48 mg phenylalanine per 3.75 gram dose.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Primary Hyperlipidemia: Colesevelam hydrochloride, the active pharmaceutical ingredient in WELCHOL, is a non-absorbed, lipid-lowering polymer that binds bile acids in the intestine, impeding their reabsorption. As the bile acid pool becomes depleted, the hepatic enzyme, cholesterol 7-α-hydroxylase, is upregulated, which increases the conversion of cholesterol to bile acids. This causes an increased demand for cholesterol in the liver cells, resulting in the dual effect of increasing transcription and activity of the cholesterol biosynthetic enzyme, HMG-CoA reductase, and increasing the number of hepatic LDL receptors. These compensatory effects result in increased clearance of LDL-C from the blood, resulting in decreased serum LDL-C levels. Serum TG levels may increase or remain unchanged.
12.2 Pharmacodynamics
A maximum therapeutic response to the lipid-lowering effects of WELCHOL was achieved within 2 weeks and was maintained during long-term therapy. In the diabetes clinical studies, a therapeutic response to WELCHOL, as reflected by a reduction in hemoglobin A1C (A1C), was initially noted following 4-6 weeks of treatment and reached maximal or near-maximal effect after 12-18 weeks of treatment.
12.3 Pharmacokinetics
Absorption: Colesevelam hydrochloride is a hydrophilic, water-insoluble polymer that is not hydrolyzed by digestive enzymes and is not absorbed.
Distribution: Colesevelam hydrochloride is not absorbed, and therefore, its distribution is limited to the gastrointestinal tract.
Metabolism: Colesevelam hydrochloride is not metabolized systemically and does not interfere with systemic drug-metabolizing enzymes such as cytochrome P-450.
Excretion: In 16 healthy volunteers, an average of 0.05% of administered radioactivity from a single 14C-labeled colesevelam hydrochloride dose was excreted in the urine.
Drug Interactions: Drug interactions between colesevelam and concomitantly administered drugs were screened through in vitro studies and confirmed in in vivo studies. In vitro studies demonstrated that cephalexin, metformin, and ciprofloxacin had negligible binding to colesevelam hydrochloride. Therefore, an in vivo pharmacokinetic interaction of WELCHOL with these drugs is unlikely. WELCHOL was found to have no significant effect on the bioavailability of digoxin, fenofibrate, lovastatin, metoprolol, quinidine, valproic acid, pioglitazone, and warfarin. The results of additional in vivo drug interactions of WELCHOL are presented in Table 5.
Drug interactions between WELCHOL and other commonly co-administered drugs in patients with type 2 diabetes (including rosiglitazone maleate, glimepiride, glipizide, sitagliptin phosphate, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, sustained-release formulations of anti-diabetic and anti-hypertensive drugs, and aspirin) have not been evaluated.
| a With verapamil, the dose of WELCHOL was 4.5 g | |||||||
| b Should be administered at least 4 hours prior to WELCHOL. [See Drug Interactions (7)] | |||||||
| * Oral contraceptive containing norethindrone and ethinyl estradiol. | |||||||
| N/A — Not Available | |||||||
|
Drug |
Dose |
Co-administered |
1 hr prior to WELCHOL |
4 hr prior to WELCHOL |
|||
| AUC0-∞ Cmax | AUC0-∞ Cmax | AUC0-∞ Cmax | |||||
| Verapamil sustained-release | 240 mg | -31% | -11% | N/A | N/A | N/A | N/A |
| Glyburideb | 3 mg | -32% | -47% | -20% | -15% | -7% | 4% |
| Levothyroxineb | 600 µg | -22% | -33% | 6% | -2% | 1% | 8% |
| Norethindrone*b | 1 mg | -1% | -20% | 5% | -3% | 6% | 7% |
| Ethinyl Estradiol*b | 0.035 mg | -24% | -24% | -18% | -1% | -12% | 0% |
| Repaglinide | 2 mg | -7% | -19% | -6% | -1% | N/A | N/A |
| Cyclosporine | 200 mg | -34% | -44% | N/A | N/A | N/A | N/A |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: A 104-week carcinogenicity study with colesevelam hydrochloride was conducted in CD-1 mice, at oral dietary doses up to 3 g/kg/day. This dose was approximately 50 times the maximum recommended human dose of 4.5 g/day, based on body weight, mg/kg. There were no significant drug-induced tumor findings in male or female mice. In a 104-week carcinogenicity study with colesevelam hydrochloride in Harlan Sprague-Dawley rats, a statistically significant increase in the incidence of pancreatic acinar cell adenoma was seen in male rats at doses >1.2 g/kg/day (approximately 20 times the maximum human dose, based on body weight, mg/kg) (trend test only). A statistically significant increase in thyroid C-cell adenoma was seen in female rats at 2.4 g/kg/day (approximately 40 times the maximum human dose, based on body weight, mg/kg).
Mutagenesis: Colesevelam hydrochloride and 4 degradants present in the drug substance have been evaluated for mutagenicity in the Ames test and a mammalian chromosomal aberration test. The 4 degradants and an extract of the parent compound did not exhibit genetic toxicity in an in vitro bacterial mutagenesis assay in S.typhimurium and E. coli (Ames assay) with or without rat liver metabolic activation. An extract of the parent compound was positive in the Chinese Hamster Ovary (CHO) cell chromosomal aberration assay in the presence of metabolic activation and negative in the absence of metabolic activation. The results of the CHO cell chromosomal aberration assay with 2 of the 4 degradants, decylamine HCl and aminohexyltrimethyl ammonium chloride HCl, were equivocal in the absence of metabolic activation and negative in the presence of metabolic activation. The other 2 degradants, didecylamine HCl and 6-decylamino-hexyltrimethyl ammonium chloride HCl, were negative in the presence and absence of metabolic activation.
13.2 Animal Toxicology and/or Pharmacology
Reproductive Toxicology Studies
Reproduction studies have been performed in rats and rabbits at doses up to 3 g/kg/day and 1 g/kg/day, respectively (approximately 50 and 17 times the maximum human dose, based on body weight, mg/kg) and have revealed no evidence of harm to the fetus due to colesevelam hydrochloride.
14 CLINICAL STUDIES
14.1 Primary Hyperlipidemia
WELCHOL reduces TC, LDL-C, apolipoprotein B (Apo B), and non-HDL-C when administered alone or in combination with a statin in patients with primary hyperlipidemia.
Approximately 1600 patients were studied in 9 clinical trials with treatment durations ranging from 4 to 50 weeks. With the exception of one open-label, uncontrolled, long-term extension study, all studies were multicenter, randomized, double-blind, and placebo-controlled. A maximum therapeutic response to WELCHOL was achieved within 2 weeks and was maintained during long-term therapy.
Monotherapy: In a study in patients with LDL-C between 130 mg/dL and 220 mg/dL (mean 158 mg/dL), WELCHOL was given for 24 weeks in divided doses with the morning and evening meals.
As shown in Table 6, the mean LDL-C reductions were 15% and 18% at the 3.8 g and 4.5 g doses. The respective mean TC reductions were 7% and 10%. The mean Apo B reductions were 12% in both treatment groups. WELCHOL at both doses increased HDL-C by 3%. Increases in TG of 9-10% were observed at both WELCHOL doses but the changes were not statistically different from placebo.
| * p<0.05 for lipid parameters compared to placebo, for Apo B compared to baseline. | |||||||
| a Median % change from baseline. | |||||||
| Grams/Day | N | TC | LDL-C | Apo B | HDL-Ca | Non-HDL-C | TGa |
| Placebo | 88 | +1 | 0 | 0 | -1 | +1 | +5 |
| 3.8 g (6 tablets) | 95 | -7* | -15* | -12* | +3* | -10* | +10 |
| 4.5 g (7 tablets) | 94 | -10* | -18* | -12* | +3 | -13* | +9 |
In a study in 98 patients with LDL-C between 145 mg/dL and 250 mg/dL (mean 169 mg/dL), WELCHOL 3.8 g was given for 6 weeks as a single dose with breakfast, as a single dose with dinner, or as divided doses with breakfast and dinner. The mean LDL-C reductions were 18%, 15%, and 18% for the 3 dosing regimens, respectively. The reductions with these 3 regimens were not statistically different from one another.
Combination Therapy: Co-administration of WELCHOL and a statin (atorvastatin, lovastatin, or simvastatin) in 3 clinical studies demonstrated an additive reduction of LDL-C. The mean baseline LDL-C was 184 mg/dL in the atorvastatin study (range 156-236 mg/dL), 171 mg/dL in the lovastatin study (range 115-247 mg/dL), and 188 mg/dL in the simvastatin study (range 148-352 mg/dL). As demonstrated in Table 7, WELCHOL doses of 2.3 g to 3.8 g resulted in an additional 8% to 16% reduction in LDL-C above that seen with the statin alone.
| *p<0.05 for lipid parameters compared to placebo, for Apo B compared to baseline. | |||||||
| a Median % change from baseline. | |||||||
| Dose/Day | N | TC | LDL-C | Apo B | HDL-Ca | Non-HDL-C | TGa |
| Atorvastatin Trial (4-week) | |||||||
| Placebo | 19 | +4 | +3 | -3 | +4 | +4 | +10 |
| Atorvastatin 10 mg | 18 | -27* | -38* | -32* | +8 | -35* | -24* |
| WELCHOL 3.8 g/Atorvastatin 10 mg | 18 | -31* | -48* | -38* | +11 | -40* | -1 |
| Atorvastatin 80 mg | 20 | -39* | -53* | -46* | +6 | -50* | -33* |
| Simvastatin Trial (6-week) | |||||||
| Placebo | 33 | -2 | -4 | -4* | -3 | -2 | +6* |
| Simvastatin 10 mg | 35 | -19* | -26* | -20* | +3* | -24* | -17* |
| WELCHOL 3.8 g/Simvastatin 10 mg | 34 | -28* | -42* | -33* | +10* | -37* | -12* |
| Simvastatin 20 mg | 39 | -23* | -34* | -26* | +7* | -30* | -12* |
| WELCHOL 2.3 g/Simvastatin 20 mg | 37 | -29* | -42* | -32* | +4* | -37* | -12* |
| Lovastatin Trial (4-week) | |||||||
| Placebo | 26 | +1 | 0 | 0 | +1 | +1 | +1 |
| Lovastatin 10 mg | 26 | -14* | -22* | -16* | +5 | -19* | 0 |
| WELCHOL 2.3 g/Lovastatin 10 mg Together | 27 | -21* | -34* | -24* | +4 | -27* | -1 |
| WELCHOL 2.3 g/Lovastatin 10 mg Apart | 23 | -21* | -32* | -24* | +2 | -28* | -2 |
In all 3 studies, the LDL-C reduction achieved with the combination of WELCHOL and any given dose of statin therapy was statistically superior to that achieved with WELCHOL or that dose of the statin alone. The LDL-C reduction with atorvastatin 80 mg was not statistically significantly different from the combination of WELCHOL 3.8 g and atorvastatin 10 mg.
The effect of WELCHOL when added to fenofibrate was assessed in 122 patients with mixed hyperlipidemia (Fredrickson Type IIb). Inclusion in the study required LDL-C ≥115 mg/dL and TG 150 mg/dL to 749 mg/dL. Patients were treated with 160 mg of fenofibrate during an 8-week open-label run-in period and then randomly assigned to receive fenofibrate 160 mg plus either WELCHOL 3.8 g or placebo for 6 weeks of double-blind treatment. The overall mean LDL-C at the start of randomized treatment was 144 mg/dL. The results of the study are summarized in Table 8.
| *p≤0.0002 compared to placebo. | |||||||
| a For triglycerides, median % change from baseline. | |||||||
| b Treated Baseline: following 8-week treatment with open-label fenofibrate 160 mg. | |||||||
| Treatment | N | TC | LDL-C | Apo B | HDL-C | Non-HDL-C | TGa |
| Placebo + Fenofibrate 160 mg | 61 | 2 | 2 | 1 | -1 | 2 | -3 |
| WELCHOL + Fenofibrate 160 mg | 61 | -6* | -10* | -7* | 0 | -8* | 6 |
Pediatric Therapy: The safety and efficacy of WELCHOL in pediatric patients were evaluated in an 8-week, multi-center, randomized, double-blind, placebo-controlled, parallel group study followed by an open-label phase, in 194 boys and postmenarchal girls 10-17 years of age (mean age 14.1 years) with heterozygous familial hypercholesterolemia (heFH), taking a stable dose of an FDA-approved statin (with LDL-C >130 mg/dL) or naïve to lipid lowering therapy (with LDL-C >160 mg/dL). This study had 3 periods: a single-blind, placebo stabilization period; an 8-week, randomized, double-blind, parallel-group, placebo controlled treatment period; and an 18-week, open-label treatment period. Forty-seven (24%) patients were taking statins and 147 (76%) patients were statin-naïve at screening. The mean baseline LDL-C at Day 1 was approximately 199 mg/dL.
During the double-blind treatment period, patients were assigned randomly to treatment: WELCHOL 3.8 g/day (n=64), WELCHOL 1.9 g/day (n=65), or placebo (n=65). In total, 186 patients completed the double-blind treatment period. After 8 weeks of treatment, WELCHOL 3.8 g/day significantly decreased plasma levels of LDL-C, non-HDL-C, TC, and Apo B and significantly increased HDL-C. A moderate, non statistically significant increase in TG was observed versus placebo (Table 9).
| *p≤0.05 for lipid parameters compared to placebo | ||||||
| Values represent LS mean. Only patients with values at both study baseline and endpoint are included in this table. Study baseline was defined as the last value measured before or on Day 1 prior to the first dose of randomized study medication. | ||||||
| a For triglycerides, median % change from baseline. | ||||||
| Results were based on the ITT population with LOCF | ||||||
| Treatment Difference | TC (N=128) | LDL-C (N=128) | Apo B (N=124) | HDL-C (N=128) | Non-HDL-C (N=128) | TGa
(N=128) |
| WELCHOL 3.8 g vs Placebo | -7* | -13* | -8* | +6* | -11* | +5 |
During the open-label treatment period patients were treated with WELCHOL 3.8 g/day. In total, 173 (89%) patients completed 26 weeks of treatment. Results at Week 26 were consistent with those at Week 8.
14.2 Type 2 Diabetes Mellitus
WELCHOL has been studied in combination with metformin, sulfonylureas, and insulin. WELCHOL has not been studied as monotherapy.
The efficacy of WELCHOL 3.8 g/day in patients with type 2 diabetes mellitus was evaluated in 3 double-blind, placebo-controlled add-on therapy trials involving a total of 1018 patients with baseline A1C 7.5-9.5%. Patients were enrolled and maintained on their pre-existing, stable, background anti-diabetic regimen. WELCHOL and placebo were administered either as 3 tablets twice daily with lunch and dinner or as 6 tablets with dinner alone.
In these studies, the overall mean age was 57 years (range 24-81 years), 47% were women, and 59% of the patients were Caucasian, 23% were Hispanic, 14% were Black, 3% were Asian, and 1% were of other racial groups. Statin use at baseline was reported in 42% of the WELCHOL-treated patients and 50% of the placebo-treated patients.
In all 3 pivotal add-on therapy trials, treatment with WELCHOL resulted in a statistically significant reduction in A1C of 0.5% compared to placebo. Similar placebo-corrected reductions in A1C occurred in patients who received WELCHOL in combination with metformin, sulfonylurea, or insulin monotherapy or combinations of these therapies with other anti-diabetic agents. In the metformin and sulfonylurea trials, treatment with WELCHOL also resulted in statistically significant reductions in fasting plasma glucose (FPG) of 14 mg/dL compared to placebo.
WELCHOL had consistent effects on A1C across subgroups of age, gender, race, body mass index, and baseline A1C. WELCHOL’s effects on A1C were also similar for the two dosing regimens (3 tablets with lunch and with dinner or 6 tablets with dinner alone).
The mean baseline LDL-C was 104 mg/dL in the metformin study (range 32-214 mg/dL), 106 mg/dL in the sulfonylurea study (range 41-264 mg/dL), and 102 mg/dL in the insulin study (range 35-204 mg/dL). In these trials, WELCHOL treatment was associated with a 12% to 16% reduction in LDL-C levels. The percentage decreases in LDL-C were of similar magnitude to those observed in patients with primary hyperlipidemia. WELCHOL treatment was associated with statistically significant increases in TG levels in the studies of patients on insulin and patients on a sulfonylurea, but not in the study of patients on metformin. The clinical significance of these increases is unknown. WELCHOL is contraindicated in patients with TG levels > 500 mg/dL [See Contraindications (4)] and periodic monitoring of lipid parameters including TG and non-HDL-C levels is recommended [See Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
Body weight did not significantly increase from baseline with WELCHOL therapy, compared with placebo, in any of the 3 pivotal clinical studies.
Add-on Combination Therapy with Metformin: WELCHOL 3.8 g/day or placebo was added to background anti-diabetic therapy in a 26-week trial of 316 patients already receiving treatment with metformin alone (N=159) or metformin in combination with other oral agents (N=157). A total of 60% of these patients were receiving ≥1,500 mg/day of metformin. In combination with metformin, WELCHOL resulted in statistically significant placebo-corrected reductions in A1C and FPG (Table 10). WELCHOL also reduced TC, LDL-C, Apo B, and non-HDL-C (Table 11). The mean percent change in serum LDL-C levels with WELCHOL compared to placebo was -16% among statin users and statin non-users; the median percent change in serum TG levels with WELCHOL compared to placebo was -2% among statin users and 10% among statin non-users. The mean change in body weight was -0.5 kg for WELCHOL and -0.3 kg for placebo.
| a Least-squares mean change calculated from an Analysis of Covariance model. | ||||||
| A1C = hemoglobin A1C, FPG = fasting plasma glucose | ||||||
|
Total Patient Population |
Metformin Alone | Metformin in Combination with Other Oral Anti-Diabetic Agents |
||||
| WELCHOL 3.8 g/day |
Placebo | WELCHOL 3.8 g/day |
Placebo | WELCHOL 3.8 g/day |
Placebo |
|
| A1C (%), Mean | ||||||
| N | 148 | 152 | 79 | 76 | 69 | 76 |
| Baseline | 8.1 | 8.1 | 8.2 | 8.2 | 8.1 | 8.0 |
| Change from baselinea | -0.4 | 0.2 | -0.4 | 0.0 | -0.4 | 0.3 |
| Treatment difference (p-value) | -0.5 (p<0.001) | -0.5 (p=0.002) | -0.6 (p<0.001) | |||
| FPG (mg/dL), Mean | ||||||
| N | 149 | 152 | 79 | 76 | 70 | 76 |
| Baseline | 178 | 174 | 184 | 180 | 171 | 168 |
| Change from baselinea | -3 | 11 | -7 | 8 | 0 | 13 |
| Treatment difference (p-value) | -14 (p=0.01) | -14 (p=0.07) | -14 (p=0.10) | |||
| *p<0.001 for lipid parameters compared to placebo (this more stringent criterion for statistical significance accounts for multiplicity testing of the lipid parameters, which were secondary endpoints in the diabetes trials) | |||||||
| a Median % change from baseline. | |||||||
| †The number of patients with analyzable data, i.e., a baseline and post-treatment value (last-observation carried forward), varied slightly among different parameters. The N given represents the smallest number of patients included in the analysis for any parameter. | |||||||
| Dose/Day | N† | TC | LDL-C | Apo B | HDL-C | Non-HDL-C | TGa |
| Total Patient Population | |||||||
| WELCHOL 3.8 g | 125 | -4* | -12* | -4* | 1 | -6* | 12 |
| Placebo | 126 | 3 | 4 | 4 | 0 | 5 | 7 |
| Metformin Alone | |||||||
| WELCHOL 3.8 g | 66 | -3 | -9 | -2 | 1 | -4 | 15 |
| Placebo | 61 | 2 | 0 | 1 | -2 | 4 | 8 |
| Metformin in Combination with Other Oral Anti-diabetic Agents | |||||||
| WELCHOL 3.8 g | 59 | -6* | -15* | -6* | 1 | -7* | 8 |
| Placebo | 65 | 4 | 7 | 7 | 2 | 6 | 5 |
Add-on Combination Therapy with Sulfonylurea: WELCHOL 3.8 g/day or placebo was added to background anti-diabetic therapy in a 26-week trial of 460 patients already treated with sulfonylurea alone (N=156) or sulfonylurea in combination with other oral agents (N=304). A total of 72% of these patients were receiving at least half-maximal doses of sulfonylurea therapy. In combination with a sulfonylurea, WELCHOL resulted in statistically significant placebo-corrected reductions in A1C and FPG (Table 12). WELCHOL also reduced TC, LDL-C, Apo B, and non-HDL-C, but increased serum TG (Table 13). The mean percent change in serum LDL-C levels with WELCHOL compared to placebo was -18% among statin users and -15% among statin non-users; the median percent increase in serum TG with WELCHOL compared to placebo was 29% among statin users and 9% among statin non-users. The mean change in body weight was 0.0 kg for WELCHOL and -0.4 kg for placebo.
| a Least-squares mean change calculated from an Analysis of Covariance model. | ||||||
| A1C = hemoglobin A1C, FPG = fasting plasma glucose | ||||||
|
Total Patient Population |
Sulfonylurea Alone | Sulfonylurea in Combination with Other Oral Anti-Diabetic Agents |
||||
| WELCHOL 3.8 g/day |
Placebo | WELCHOL 3.8 g/day |
Placebo | WELCHOL 3.8 g/day |
Placebo |
|
| A1C (%), Mean | ||||||
| n | 218 | 218 | 69 | 80 | 149 | 138 |
| Baseline | 8.2 | 8.3 | 8.2 | 8.4 | 8.2 | 8.3 |
| Change from baselinea | -0.3 | 0.2 | -0.3 | 0.5 | -0.4 | 0.0 |
| Treatment difference (p-value) | -0.5 (p<0.001) | -0.8 (p<0.001) | -0.4 (p<0.001) | |||
| FPG (mg/dL), Mean | ||||||
| n | 218 | 217 | 70 | 80 | 148 | 137 |
| Baseline | 177 | 181 | 181 | 186 | 175 | 178 |
| Change from baselinea | -4 | 10 | 3 | 15 | -11 | 4 |
| Treatment difference (p-value) | -14 (p=0.009) | -12 (p=0.18) | -14 (p=0.03) | |||
| *p<0.001 for lipid parameters compared to placebo (this more stringent criterion for statistical significance accounts for multiplicity testing of the lipid parameters, which were secondary endpoints in the diabetes trials) | |||||||
| a Median % change from baseline. | |||||||
| †The number of patients with analyzable data, i.e., a baseline and post-treatment value (last-observation carried forward), varied slightly among different parameters. The N given represents the smallest number of patients included in the analysis for any parameter. | |||||||
| Dose/Day | N† | TC | LDL-C | Apo B | HDL-C | Non-HDL-C | TGa |
| Total Patient Population | |||||||
| WELCHOL 3.8 g | 186 | -5* | -16* | -6* | 1 | -6* | 20* |
| Placebo | 193 | 0 | 1 | 1 | 0 | 1 | 1 |
| Sulfonylurea Alone | |||||||
| WELCHOL 3.8 g | 57 | -5 | -14* | -5 | -1 | -6 | 17 |
| Placebo | 68 | 0 | 1 | 1 | 1 | 0 | -1 |
| Sulfonylurea in Combination with Other Oral Anti-diabetic Agents | |||||||
| WELCHOL 3.8 g | 129 | -5 | -18* | -7* | 1 | -6 | 21* |
| Placebo | 125 | 0 | 0 | 1 | 0 | 1 | 2 |
Add-on Combination Therapy with Insulin: WELCHOL 3.8 g/day or placebo was added to background anti-diabetic therapy in a 16-week trial of 287 patients already treated with insulin alone (N=116) or insulin in combination with oral agents (N=171). At baseline, the median daily insulin dose was 70 units in the WELCHOL group and 65 units in the placebo group. In combination with insulin, WELCHOL resulted in a statistically significant placebo-corrected reduction in A1C (Table 14). WELCHOL also reduced LDL-C and Apo B, but increased serum TG (Table 15). The mean percent change in serum LDL-C levels with WELCHOL compared to placebo was -13% among statin users and statin non-users; the median percent increase in serum TG levels with WELCHOL compared to placebo was 24% among statin users and 17% among statin non-users. The mean change in body weight was 0.6 kg for WELCHOL and 0.2 kg for placebo.
| a Least-squares mean change calculated from an Analysis of Covariance model. | ||||||
| A1C = hemoglobin A1C, FPG = fasting plasma glucose | ||||||
|
Total Patient Population |
Insulin Alone | Insulin in Combination with Other Oral Anti-Diabetic Agents |
||||
| WELCHOL 3.8 g/day |
Placebo | WELCHOL 3.8 g/day |
Placebo | WELCHOL 3.8 g/day |
Placebo |
|
| A1C (%), Mean | ||||||
| n | 144 | 136 | 54 | 55 | 90 | 81 |
| Baseline | 8.3 | 8.2 | 8.2 | 8.3 | 8.3 | 8.2 |
| Change from baselinea | -0.4 | 0.1 | -0.4 | 0.2 | -0.4 | 0.0 |
| Treatment difference (p-value) | -0.5 (p<0.001) | -0.6 (p<0.001) | -0.4 (p<0.001) | |||
| FPG (mg/dL), Mean | ||||||
| n | 144 | 136 | 54 | 55 | 90 | 81 |
| Baseline | 165 | 151 | 165 | 163 | 165 | 143 |
| Change from baselinea | 2 | 16 | 8 | 17 | -4 | 14 |
| Treatment difference (p-value) | -15 (p=0.08) | -9 (p=0.51) | -18 (p=0.09) | |||
| *p<0.001 for lipid parameters compared to placebo (this more stringent criterion for statistical significance accounts for multiplicity testing of the lipid parameters, which were secondary endpoints in the diabetes trials) | |||||||
| a Median % change from baseline. | |||||||
| †The number of patients with analyzable data, i.e., a baseline and post-treatment value (last-observation carried forward), varied slightly among different parameters. The N given represents the smallest number of patients included in the analysis for any parameter. | |||||||
| Dose/Day | N† | TC | LDL-C | Apo B | HDL-C | Non-HDL-C | TGa |
| Total Patient Cohort | |||||||
| WELCHOL 3.8 g | 129 | -3 | -12* | -4 | -1 | -3 | 23* |
| Placebo | 121 | 1 | 1 | 1 | 0 | 1 | 0 |
| Insulin Alone | |||||||
| WELCHOL 3.8 g | 46 | -3 | -12 | -5 | 0 | -3 | 19 |
| Placebo | 48 | 2 | 4 | 2 | 3 | 2 | -2 |
| Insulin in Combination with Other Oral Anti-diabetic Agents | |||||||
| WELCHOL 3.8 g | 83 | -4 | -13 | -4 | -1 | -3 | 25* |
| Placebo | 73 | -1 | -3 | 0 | -1 | -1 | 2 |
16 HOW SUPPLIED/STORAGE AND HANDLING
WELCHOL (colesevelam hydrochloride) Tablets, 625 mg, are supplied as an off-white, solid tablet imprinted with the word “Sankyo” and “C01” on one side. WELCHOL tablets are available as follows:
• Bottles of 180 - NDC 21695-781-78
Storage: Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Brief exposure to 40°C (104°F) does not adversely affect the product. Protect from moisture.
WELCHOL (colesevelam hydrochloride) for Oral Suspension is a white to pale yellow powder containing yellow granules. WELCHOL for Oral Suspension is available as follows:
- 3.75 gram single-dose packet
Cartons of 30 packets – NDC 21695-779-30
Storage: Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Protect from moisture.
17 PATIENT COUNSELING INFORMATION
Dosing: Patients should be advised to take WELCHOL Tablets with a meal and liquid. WELCHOL can be taken as 6 tablets once daily or 3 tablets twice daily. Patients should be advised to take WELCHOL for Oral Suspension as one 3.75 gram packet once daily or one 1.875 gram packet twice daily. To prepare, empty the entire contents of one packet into a glass or cup. Add ½ to 1 cup (4 to 8 ounces) of water. Stir well and drink. WELCHOL for Oral Suspension should be taken with meals. To avoid esophageal distress, WELCHOL for Oral Suspension should not be taken in its dry form. Always mix WELCHOL for Oral Suspension with water before ingesting [See Dosage and Administration(2)]
Drug interactions: Drugs with a known interaction with colesevelam (e.g., glyburide, levothyroxine, oral contraceptives) should be administered at least 4 hours prior to WELCHOL. Drugs that have not been tested for interaction with colesevelam, especially those with a narrow therapeutic index (e.g., phenytoin), should also be administered at least 4 hours prior to WELCHOL. Alternatively the physician should monitor blood levels of the coadministered drug. [See Drug Interactions (7)]
Gastrointestinal: WELCHOL can cause constipation. WELCHOL is contraindicated in patients with a history of bowel obstruction. WELCHOL is not recommended in patients who may be at risk of bowel obstruction, including patients with gastroparesis, other gastrointestinal motility disorders, or a history of major gastrointestinal surgery. Patients should be instructed to consume a diet that promotes bowel regularity. Patients should be instructed to promptly discontinue WELCHOL and seek medical attention if severe abdominal pain or severe constipation occurs. Because of the tablet size, WELCHOL Tablets can cause dysphagia or esophageal obstruction and should be used with caution in patients with dysphagia or swallowing disorders. To avoid esophageal distress, WELCHOL for Oral Suspension should not be taken in its dry form. Always mix WELCHOL for Oral Suspension with water before ingesting [See Warnings and Precautions (5.4)]
Hypertriglyceridemia and pancreatitis: Patients should be instructed to discontinue WELCHOL and seek prompt medical attention if the hallmark symptoms of acute pancreatitis occur (e.g., severe abdominal pain with or without nausea and vomiting). [See Warnings and Precautions (5.2)]
17.1 Primary Hyperlipidemia:
Patients should be advised to adhere to their National Cholesterol Education Program (NCEP)-recommended diet.
17.2 Type 2 Diabetes Mellitus:
General: Patients should be advised that it is important to adhere to dietary instructions, a regular exercise program, and regular testing of blood glucose.
Hypertriglyceridemia and cardiovascular disease: Patients receiving a sulfonylurea or insulin should be informed that WELCHOL may increase serum triglyceride concentrations and that the long-term effect of hypertriglyceridemia on the risk of coronary artery disease is uncertain. [See Warnings and Precautions (5.2)]
Marketed by: Daiichi Sankyo, Inc
Parsippany, New Jersey 07054
Active Ingredient: Product of Austria
P1801115
Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320