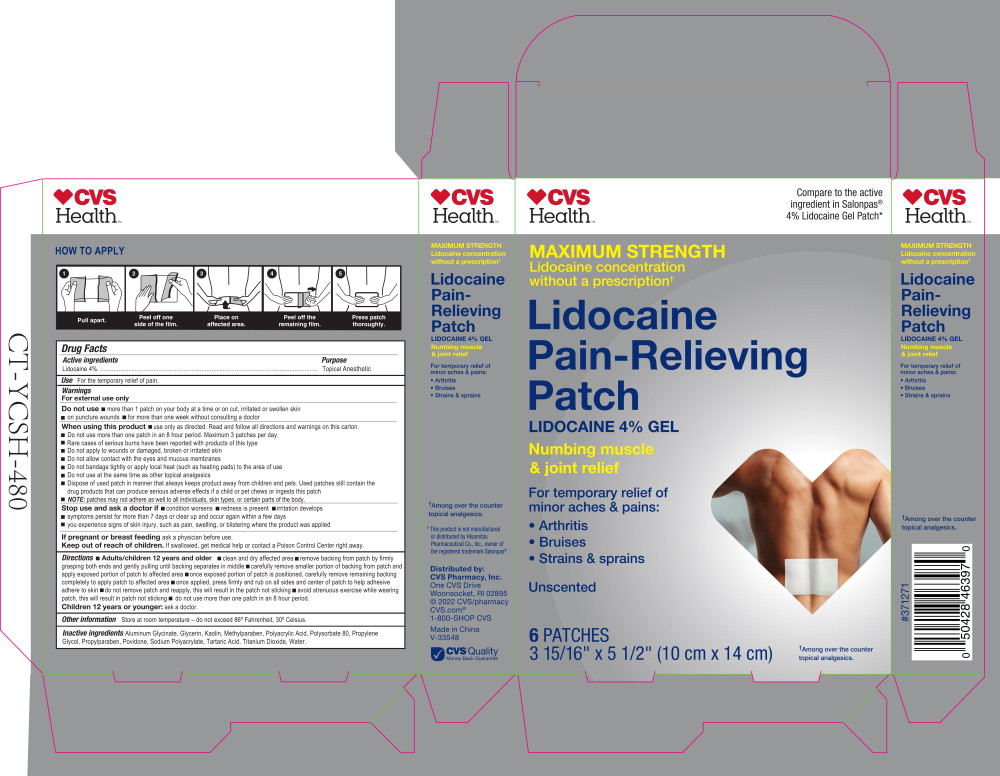
Warnings
For external use only
Do not use
- more than 1 patch on your body at a time or on cut, irritated or swollen skin
- on puncture wounds
- for more than one week without consulting a doctor
When using this product
- use only as directed. Read and follow all directions and warnings on this carton.
- Do not use more than one patch in an 8 hour period. Maximum 3 patches per day.
- Rare cases of serious burns have been reported with products of this type
- Do not apply to wounds or damaged, broken or irritated skin
- Do not allow contact with the eyes and mucous membranes
- Do not bandage tightly or apply local heat (such as heating pads) to the area of use
- Do not use at the same time as other topical analgesics
- Dispose of used patch in manner that always keeps product away from children and pets. Used patches still contain the drug products that can produce serious adverse effects if a child or pet chews or ingests this patch
- NOTE: patches may not adhere as well to all individuals, skin types, or certain parts of the body.
Directions
- Adults/children 12 years and older
- clean and dry affected area
- remove backing from patch by firmly grasping both ends and gently pulling until backing separates in middle
- carefully remove smaller portion of backing from patch and apply exposed portion of patch to affected area
- once exposed portion of patch is positioned, carefully remove remaining backing completely to apply patch to affected area
- once applied, press firmly and rub on all sides and center of patch to help adhesive adhere to skin
- do not remove patch and reapply, this will result in the patch not sticking
- avoid strenuous exercise while wearing patch, this will result in patch not sticking
- do not use more than one patch in an 8 hour period.
Inactive ingredients
Aluminum Glycinate, Glycerin, Kaolin, Methylparaben, Polyacrylic Acid, Polysorbate 80, Propylene Glycol, Propylparaben, Povidone, Sodium Polyacrylate, Tartaric Acid, Titanium Dioxide, Water.
Principal Display Panel – 567 mg Carton Label
CVS
Health™
Compare to the active
ingredient in Salonpas®
4% Lidocaine Gel Patch*
MAXIMUM STRENGTH
Lidocaine concentration
without a prescription†
Lidocaine
Pain-Relieving
Patch
LIDOCAINE 4% GEL
Numbing muscle
& joint relief
For temporary relief of
minor aches & pains:
- Arthritis
- Bruises
- Strains & sprains
Unscented
6 PATCHES
3 15/16" x 5 1/2" (10 cm x 14 cm)
†Among over the counter
topical analgesics.
Principal Display Panel – 567 mg Vial Label
CVS
Health™
MAXIMUM STRENGTH
Lidocaine concentration
without a prescription†
Lidocaine
Pain-Relieving
Patch
LIDOCAINE 4% GEL
Numbing muscle & joint relief
For temporary relief of
minor aches & pains:
- Arthritis
- Bruises
- Strains & sprains
Unscented
1 PATCH
3 15/16" x 5 1/2"
(10 cm x 14 cm)
†Among over the counter
topical analgesics.