FAR AWAY DREAMS ROLL-ON ANTIPERSPIRANT DEODERANT- aluminum chlorohydrate gel
Avon Products, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
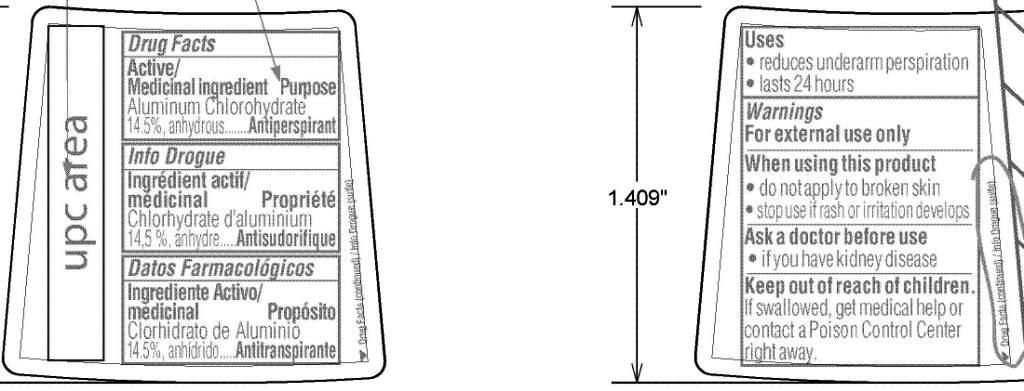
Active/
Medicinal ingredient Purpose
Aluminum Chlorohydrate
14.5%, anhydrous...................Antiperspirant
Uses
• reduces underarm perspiration
• lasts 24 hours
Warnings
For external use only
When using this product
• do not apply to broken skin
• stop use if rash or irritation develops
Ask a doctor before use
• if you have kidney disease
Keep out of reach of children.
If swallowed, get medical help or
contact a Poison Control Center
right away.
Directions
• apply to underarms only
INACTIVE / NON-MEDICINAL
INGREDIENTS :
WATER/EAU, STEARETH-2,
PPG-15 STEARYL ETHER,
DICAPRYL ADIPATE, PARFUM/
FRAGRANCE, STEARETH-20,
ISOPROPYL PALMITATE.
Questions? Call 1-800-FOR-AVON
or 1-800-265-AVON in Canada
Avon Products, Inc.