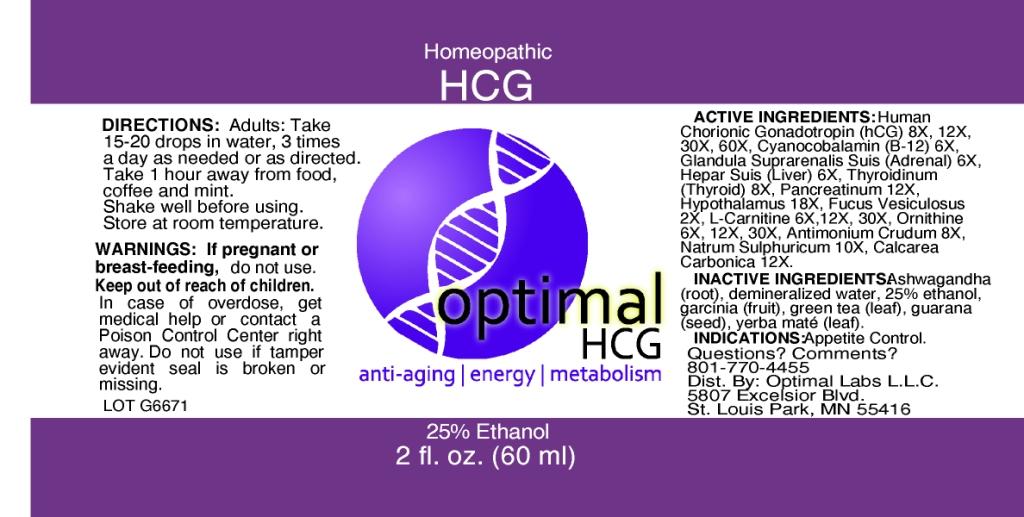
ACTIVE INGREDIENTS: Fucus vesiculosus 2X, Cyanocobalamin 6X, Glandula suprarenalis suis 6X, Hepar suis 6X, L-carnitine 6X, 12X, 30X, Ornithine 6X, 12X, 30X, Antimonium crudum 8X, Human chorionic gonadotropin(hCG) 8X, 12X, 30X, 60X, Thyroidinum 8X, Natrum sulphuricum 10X, Calcarea carbonica 12X, Pancreatinum 12X, Hypothalamus 18X.
WARNINGS: If pregnant or breast-feeding, do not use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing.
DIRECTIONS: Adults: Take 15-20 drops in water, 3 times a day as needed or as directed. Hold under tongue for 20 seconds. Take 1 hour away from food, coffee and mint. Shake well before using. Store at room temperature.
INACTIVE INGREDIENTS: Green tea (leaf), Yerba mate (leaf), Guarana (seed), Ashwagandha (root), Demineralized water, 25% Ethanol.
KEEP OUT OF REACH OF CHILDREN: In case of overdose, get medical help or contact a Poison Control Center right away.