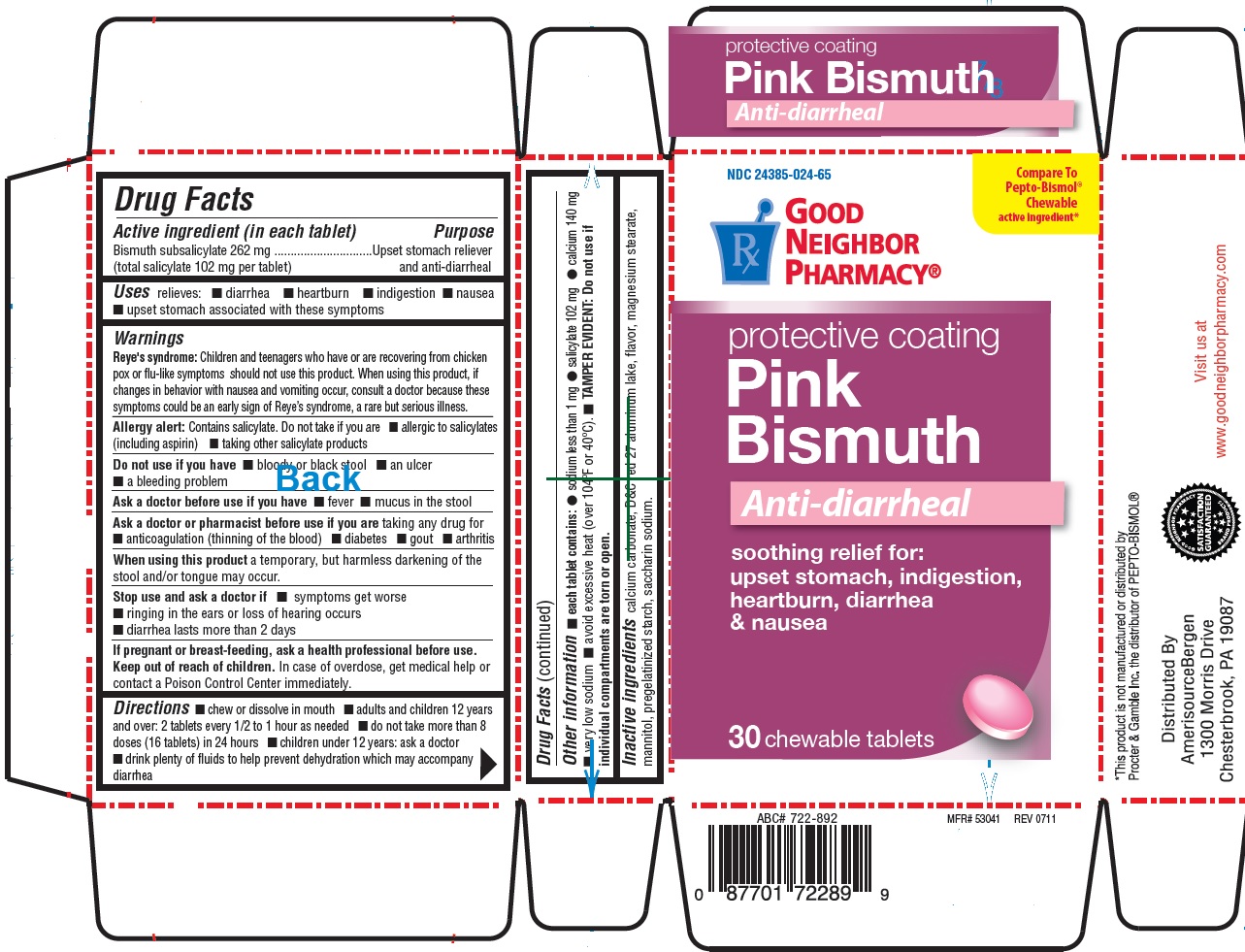
Active ingredient (in each tablet)
Bismuth subsalicylate 262 mg
(total salicylate 102 mg per tablet)
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. when using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are
allergic to salicylates (including aspirin)
taking other salicylate products
Do not use if you have
- bloody or black stools
- an ulcer
- a bleeding problem
Ask a doctor before use if you have
- fever
- mucus in the stool
Ask a doctor or pharmacist before use if you are
- taking any drug for anticoagulation (thinning of the blood)
- diabetes
- gout
- arthritis
Directions
- chew or dissolve in mouth
- adults and children 12 years and over: 2 tablets every 1/2 to 1 hour as needed
- do not take more than 8 doses (16 tablets) in 24 hours
- children under 12 years: ask a doctor
- drink plenty of fluids to help prevent dehydration which may accompany diarrhea.
Other information
- each tablet contains:sodium less than 1 mg
- salicylate 102 mg
- calcium 140 mg
- very low sodium
- avoid excessive heat (over 104˚F or 40˚C)
- TAMPER EVIDENT: Do not use if individual compartments are torn or missing.