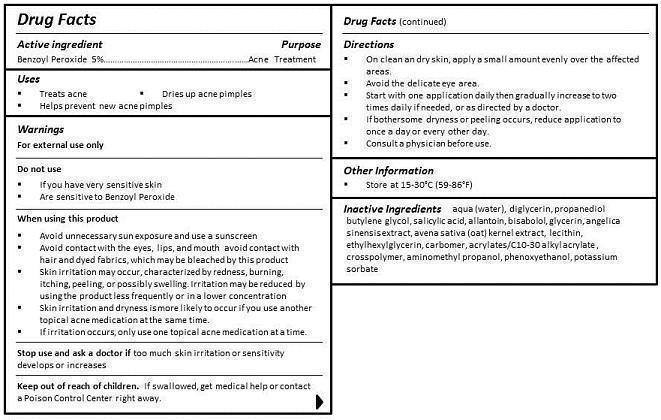
WHEN USING THIS PRODUCT
- AVOID UNNECESSARY SUN EXPOSURE AND USE A SUNSCREEN
- AVOID CONTACT WITH THE EYES, LIPS, AND MOUTH. AVOID CONTACT WITH HAIR AND DYED FABRICS, WHICH MAY BE BLEACHED BY THE PRODUCT.
- SKIN IRRITATION MAY OCCUR, CHARACTERIZED BY REDNESS, BURNING, ITCHING, PEELING, OR POSSIBLY SWELLING. IRRITATION MAY BE REDUCED BY USING THE PRODUCT LESS FREQUENTLY OR IN A LOWER CONCENTRATION.
- SKIN IRRITATION AND DRYNESS IS MORE LIKELY TO OCCUR IF YOU USE ANOTHER TOPICAL ACNE MEDICATION AT THE SAME TIME.
- IF IRRITATION OCCURS, ONLY USE ONE TOPICAL ACNE MEDICATION AT A TIME.
KEEP OUT OF REACH OF CHILDREN. IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
DIRECTIONS
- ON CLEAN AND DRY SKIN, APPLY A SMALL AMOUNT EVENLY OVER THE AFFECTED AREAS.
- AVOID THE DELICATE EYE AREA.
- START WITH ONE APPLICATION DAILY THEN GRADUALLY INCREASE TO TWO TIMES DAILY IF NEEDED, OR AS DIRECTED BY A DOCTOR.
- IF BOTHERSOME DRYNESS OR PEELING OCCURS, REDUCE APPLICATION TO ONCE A DAY OR EVERY OTHER DAY.
- CONSULT A PHYSICIAN BEFORE USE.
INGREDIENTS
aqua (water), diglycerin, propanediol, butylene glycol, salicylic acid, allantoin, bisabolol, glycerin, angelica sinensis extract, avena sativa (oat) kernel extract, lecithin, ethylhexylglycerin, carbomer, acrylates/C10-30 alkyl acrylate , crosspolymer, aminomethyl propanol, phenoxyethanol, potassium sorbate