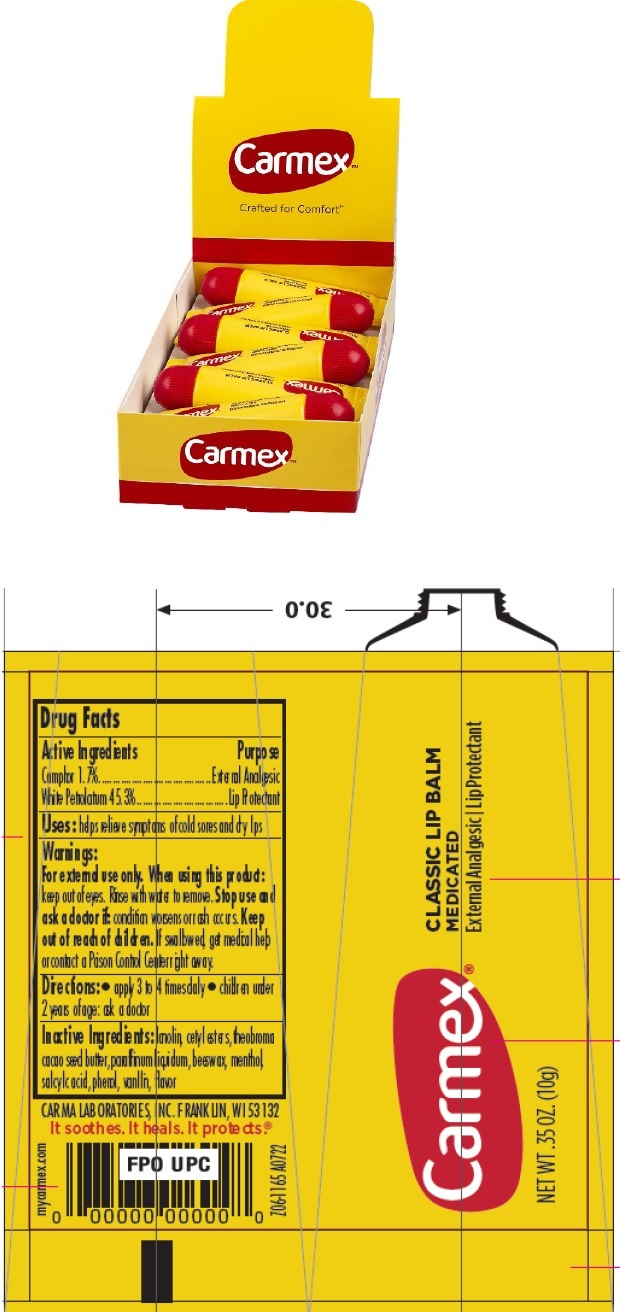
Active Ingredients
Camphor 1.70%
White Petrolatum 45.30%
Purpose
External Analgesic
Lip Protectant
Uses
- helps provide relief of symptoms of cold sores and dry lips
Warnings
For external use only
When using this product:
keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if:
- condition worsens • symptoms last more than 7 days or clear up and occur again within a few days
Do not use on:
• deep puncture wounds • serious burns • animal bites
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply to lips not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
Other Information
- this is a personal care item, and should be used by one individual only
- protect this product from excessive heat and direct sun
Inactive Ingredients
lanolin, cetyl esters, theobroma cacao seed butter, paraffin, beeswax, menthol, salicylic acid, phenol, vanillin, flavor
Package Labeling:


Package Labeling:10210-0022-7

Carma Laboratories, Inc.



