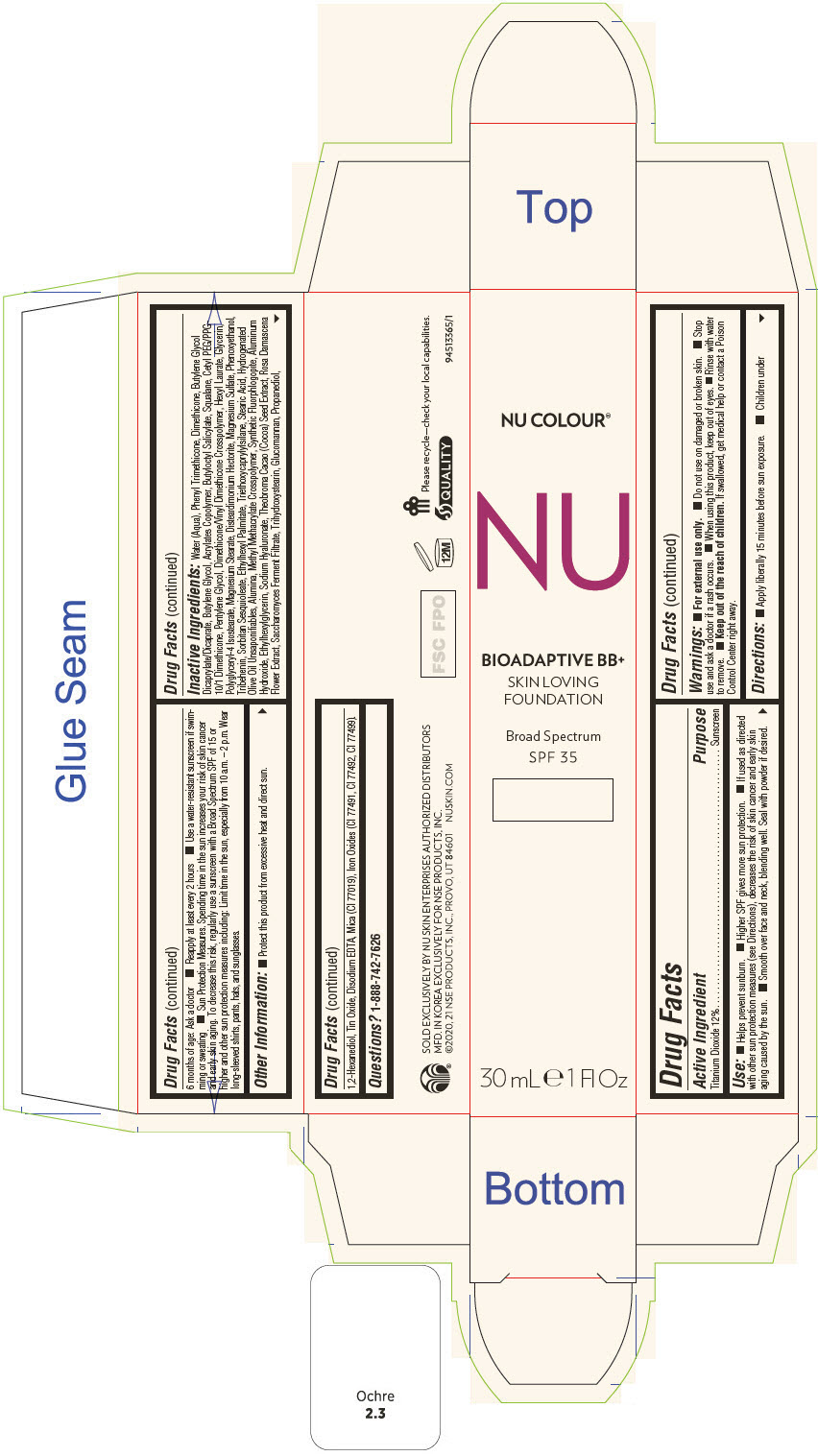
Use
- Helps prevent sunburn.
- Higher SPF gives more sun protection.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Smooth over face and neck, blending well. Seal with powder if desired.
Directions
- Apply liberally 15 minutes before sun exposure.
- Children under 6 months of age: Ask a doctor
- Reapply at least every 2 hours
- Use a water-resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures including: Limit time in the sun, especially from 10 a.m. – 2 p.m. Wear long-sleeved shirts, pants, hats, and sunglasses.
Inactive Ingredients
Water (Aqua), Phenyl Trimethicone, Dimethicone, Butylene Glycol Dicaprylate/Dicaprate, Butylene Glycol, Acrylates Copolymer, Butyloctyl Salicylate, Squalane, Cetyl PEG/PPG- 10/1 Dimethicone, Pentylene Glycol, Dimethicone/Vinyl Dimethicone Crosspolymer, Hexyl Laurate, Glycerin, Polyglyceryl-4 Isostearate, Magnesium Stearate, Disteardimonium Hectorite, Magnesium Sulfate, Phenoxyethanol, Tribehenin, Sorbitan Sesquioleate, Ethylhexyl Palmitate, Triethoxycaprylylsilane, Stearic Acid, Hydrogenated Olive Oil Unsaponifiables, Alumina, Methyl Methacrylate Crosspolymer, Synthetic Fluorphlogopite, Aluminum Hydroxide, Ethylhexylglycerin, Sodium Hyaluronate, Theobroma Cacao (Cocoa) Seed Extract, Rosa Damascena Flower Extract, Saccharomyces Ferment Filtrate, Trihydroxystearin, Glucomannan, Propanediol, 1,2-Hexanediol, Tin Oxide, Disodium EDTA, Mica (CI 77019), Iron Oxides (CI 77491, CI 77492, CI 77499).
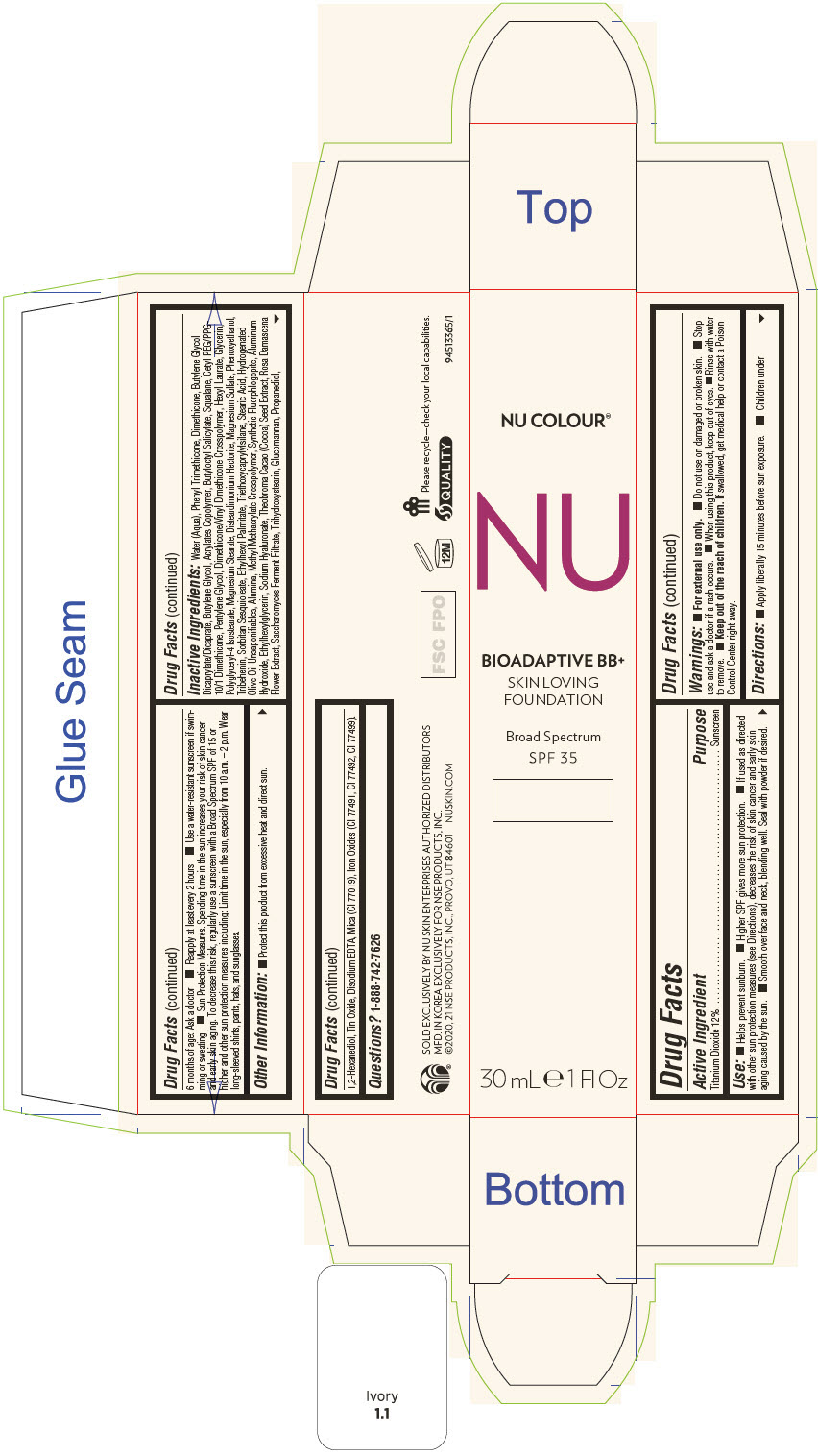
PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Ivory
NU COLOUR®
NU
BIOADAPTIVE BB+
SKIN LOVING
FOUNDATION
Broad Spectrum
SPF 35
30 mL ℮ 1 Fl Oz
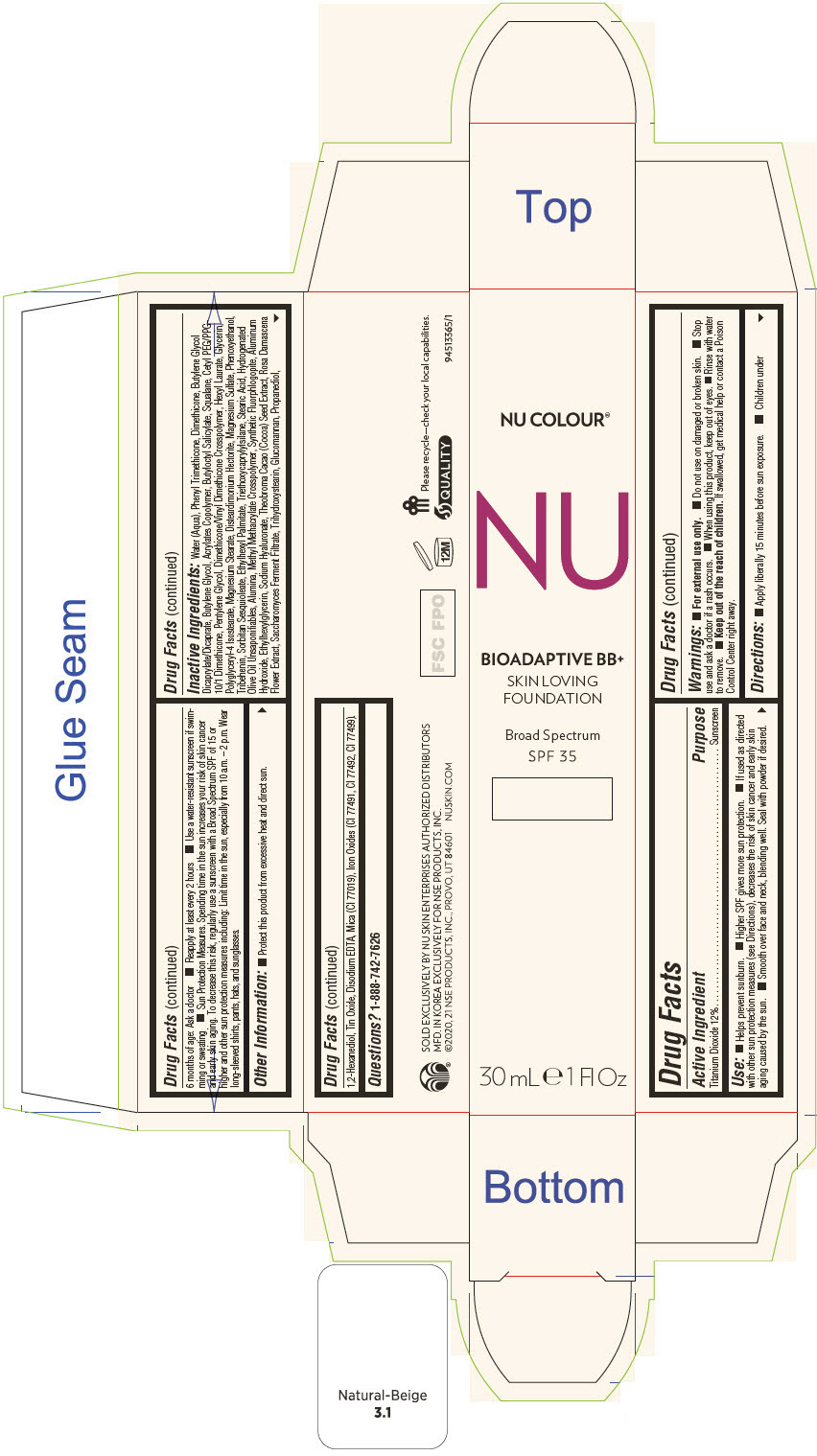
PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Linen
NU COLOUR®
NU
BIOADAPTIVE BB+
SKIN LOVING
FOUNDATION
Broad Spectrum
SPF 35
30 mL ℮ 1 Fl Oz
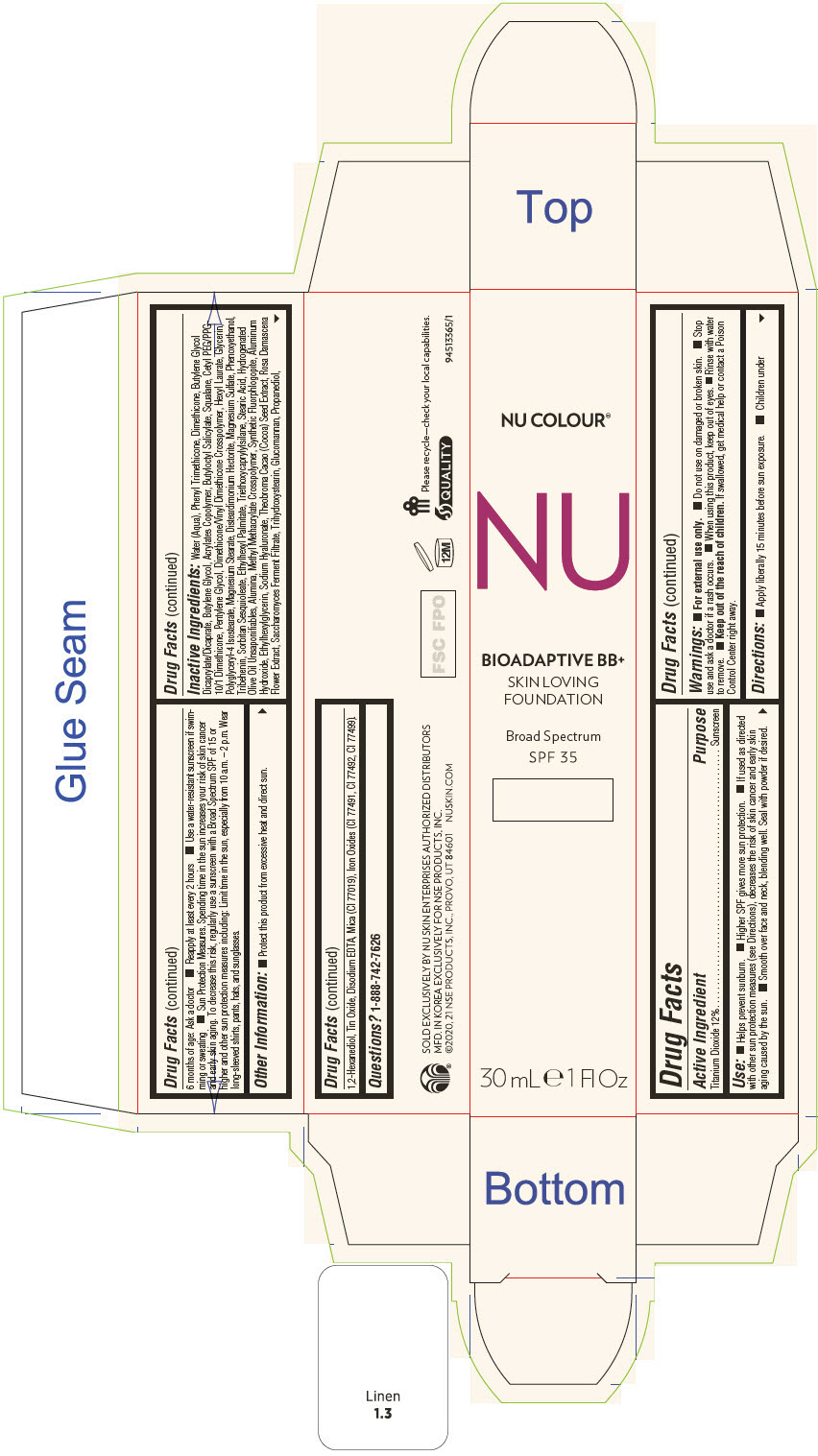
PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Ochre
NU COLOUR®
NU
BIOADAPTIVE BB+
SKIN LOVING
FOUNDATION
Broad Spectrum
SPF 35
30 mL ℮ 1 Fl Oz