BUTORPHANOL- butorphanol tartrate injection, solution
Butler Animal Health Supply, LLC dba Covetrus North America
----------
Butorphanol tartrate injection CIV
DESCRIPTION
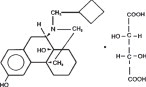
Butorphanol tartrate is a totally synthetic centrally acting, narcotic agonist-antagonist analgesic with potent antitussive activity. It is a member of the phenanthrene series. The chemical name is Morphinan-3, 14-diol, 17-(cyclobutylmethyl)-,(-)-, (S-(R*,R*))-2,3-dihydroxybutanedioate (1:1) (salt). It is a white, crystalline, water soluble substance having a molecular weight of 477.55; its molecular formula is C21H29NO2•C4H6O6.
Chemical Structure:
Each mL of butorphanol tartrate injection contains 10 mg butorphanol base (as butorphanol tartrate), 3.3 mg citric acid, 6.4 mg sodium citrate, 4.7 mg sodium chloride, and 0.1 mg benzethonium chloride, q.s, with water for injection.
CLINICAL PHARMACOLOGY
Comparative Pharmacology
In animals, butorphanol has been demonstrated to be 4 to 30 times more potent than morphine and pentazocine (Talwin®-V) respectively.1 In humans, butorphanol has been shown to have 5 to 7 times the analgesic activity of morphine and 20 times that of pentazocine.2,3 Butorphanol has 15 to 20 times the oral antitussive activity of codeine or dextromethorphan in dogs and guinea pigs.4
As an antagonist, butorphanol is approximately equivalent to nalorphine and 30 times more potent than pentazocine.1
Cardiopulmonary depressant effects are minimal after treatment with butorphanol as demonstrated in dogs,5 humans 6,7 and horses.6 Unlike classical narcotic agonist analgesics which are associated with decreases in blood pressure, reduction in heart rate, and concomitant release of histamine, butorphanol does not cause histamine release.1 Furthermore, the cardiopulmonary effects of butorphanol are not distinctly dosage related but rather reach a ceiling effect beyond which further dosage increases result in relatively lesser effects.
Reproduction: Studies performed in mice and rabbits revealed no evidence of impaired fertility or harm to the fetus due to butorphanol tartrate. In the female rat, parenteral administration was associated with increased nervousness and decreased care for the newborn, resulting in a decreased survival rate of the newborn. This nervousness was seen only in the rat species.
Equine Pharmacology
Following intravenous injection in horses, butorphanol is largely eliminated from the blood within 3 to 4 hours. The drug is extensively metabolized in the liver and excreted in the urine.
In ponies, butorphanol given intramuscularly at a dosage of 0.22 mg/kg was shown to alleviate experimentally induced visceral pain for about 4 hours.9
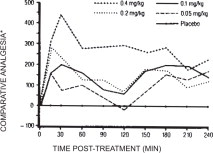
In horses, intravenous dosages of butorphanol ranging from 0.05 to 0.4 mg/kg were shown to be effective in alleviating visceral and superficial pain for at least four hours, as illustrated in the following figure:
Analgesic Effects of Butoiphanol Given at Various Dosages in Horses with Abdominal Pain
*Pain threshold in butorphanol-treated colicky horses relative to placebo controls
A definite dosage-response relationship was detected in that butorphanol dosage of 0.1 mg/kg was more effective than 0.05 mg/kg but not different from 0.2 mg/kg in alleviating deep abdominal pain.
Acute Equine Studies
Rapid intravenous administration of butorphanol at a dosage of 2.0 mg/kg (20 times the recommended dosage) to a previously unmedicated horse resulted in a brief episode of inability to stand, muscle fasciculation, a convulsive seizure of 6 seconds duration, and recovery within three minutes. The same dosage administered after 10 successive daily 1.0 mg/kg dosages of butorphanol resulted only in transient sedative effects. During the 10 day course of administration at 1.0 mg/kg (10 times the recommended use level) in two horses, the only detectable drug effects were transient behavioral changes typical of narcotic agonist activity. These included muscle fasciculation about the head and neck, dysphoria, lateral nystagmus, ataxia, and salivation. Repeated administration of butorphanol at 1.0 mg/kg (10 times the recommended dose) every four hours for 48 hours caused constipation in one of two horses.
Subacute Equine Studies
Horses were found to tolerate butorphanol given intravenously at dosages of 0.1, 0.3, and 0.5 mg/kg every 4 hours for 48 hours followed by once daily injections for a total of 21 days. The only detectable drug effects were slight transient ataxia observed occasionally in the high dosage group. No clinical, laboratory, or gross or histopathologic evidence of any butorphanol-related toxicity was encountered in the horses.
INDICATIONS
Butorphanol tartrate is indicated for the relief of pain associated with colic in adult horses and yearlings. Clinical studies in the horse have shown that butorphanol tartrate alleviates abdominal pain, associated with torsion, impaction, intussusception, spasmodic and tympanic colic, and postpartum pain.
CAUTION
Butorphanol tartrate a potent analgesic, should be used with caution with other sedative or analgesic drugs as these are likely to produce additive effects.
There are no well-controlled studies using butorphanol in breeding horses, weanlings, and foals. Therefore, the drug should not be used in these groups.
ADVERSE REACTIONS
In clinical trials in horses, the most commonly observed side effect was slight ataxia which lasted 3 to 10 minutes. Marked ataxia was reported in 1.5% of the 327 horses treated. Mild sedation was reported in 9% of the horses.
DOSAGE
The recommended dosage in the horse is 0.1 mg of butorphanol per kilogram of body weight (0.05 mg/lb) by intravenous injection. This is equivalent to 5 mL of Butorphanol tartrate for each 1000 lbs body weight. The dose may be repeated within 3 to 4 hours but treatment should not exceed 48 hours. Pre-clinical model studies and clinical field trials in horses demonstrate that the analgesic effects of butorphanol tartrate are seen within 15 minutes following injection and persist for about 4 hours.
HOW SUPPLIED
Butorphanol tartrate injection, 10 mg base activity per mL.
NDC 11695-6838-2 20 mL vial in package of one
NDC 11695-6838-5 50 mL vial in package of one
REFERENCES
- Pircio. A.W. et al: The Pharmacology of Butorphanol. Arch. Int. Pharmacodyn. Ther. 220(2):231-257,1976.
- Dobkin, A.B. et al: Butorphanol and Pentazocine in Patients with Severe Postoperative Pain. Clin. Pharmacol. Ther 18:547-553, 1975.
- Gilbert, M.S. et al: Intramuscular Butorphanol and Meperidine in Postoperative Pain. Clin. Pharmacol. Ther. 20:359-364, 1976.
- Cavanagh, R.L. et al: Antitussive Properties of Butorphanol, Arch. Int. Pharmacodyn. Ther. 220 258-268,1976.
- Shurig, J.E. et al: Effect of Butorphanol and Morphine on Pulmonary Mechanics, Arterial Blood Pressure, and Venous Plasma Histamine in the Anesthetized Dog. Arch. Int. Pharmacodyn. Ther. 233:296-304, 1978.
- Nagashmina, H. et al: Respiratory and Circulatory Effects of Intravenous Butorphanol and Morphine Clin. Pharmacol. Ther. 19:735-745, 1976.
- Popio, K.A. et al. Hemodynamic and Respiratory Effects of Morphine and Butorphanol. Clin. Pharm. Ther. 23:281-287, 1978.
- Robertson, J.T. and Muir, W.W.: Cardiopulmonary Effects of Butorphanol Tartrate in Horses. Am. J. Vef. Res. 42:41-44,1981.
- Kalpravidh, M. et al: Effects of Butorphanol, Flunixin, Levorphanol, Morphine, Pentazocine and Xylazine in Ponies. Am. J. Vet. Res. 45:217-223,1984.
Approved by FDA under ANADA 200-332
covetrus
Distributed by:
Covetrus North America
400 Metro Place North
Dublin, OH 43017
covetrus.com
AH-Butorphanol tartrate-05
HBU00N REV: 0522
Reorder #071070 for 20 mL Vial
Reorder #075515 for 50 mL Vial
Principal Display Panel Text for Container Label:
covetrus Logo
NDC: 11695-6838-2
Butorphanol CIV
tartrate injection
Contains 10 mg butorphanol base
per mL as butorphanol tartrate, USP
Caution: Federal (USA) law restricts this drug to
use by or on the order of a licensed veterinarian.
ANADA #200-332, Approved by FDA 20 mL
Principal Display Panel Text for Carton Label:
covetrus Logo
NDC: 11695-6838-2
Butorphanol
tartrate CIV
injection
Contains 10 mg butorphanol base
per mL as butorphanol tartrate, USP
Caution: Federal (USA) law restricts
this drug to use by or on the
order of a licensed veterinarian.
ANADA #200-332, Approved by FDA
20 mL
| BUTORPHANOL
butorphanol tartrate injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Butler Animal Health Supply, LLC dba Covetrus North America (603750329) |
| Registrant - Akorn Operating Company LLC (117693100) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn | 117696790 | PACK, LABEL | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn | 117696832 | MANUFACTURE, ANALYSIS, STERILIZE | |