PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
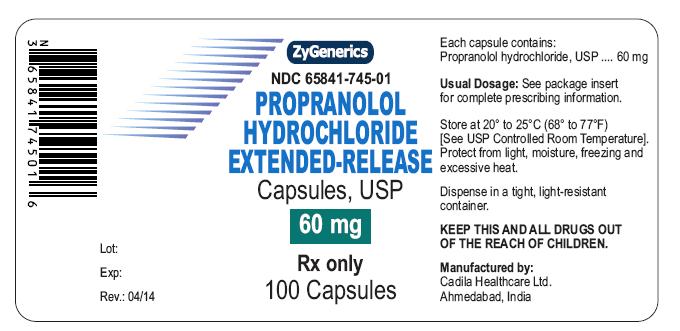
NDC 65841-745-01 in bottle of 100 Capsules
Propranolol Hydrochloride Extended-release Capsules USP, 60 mg
Rx only
100 CAPSULES
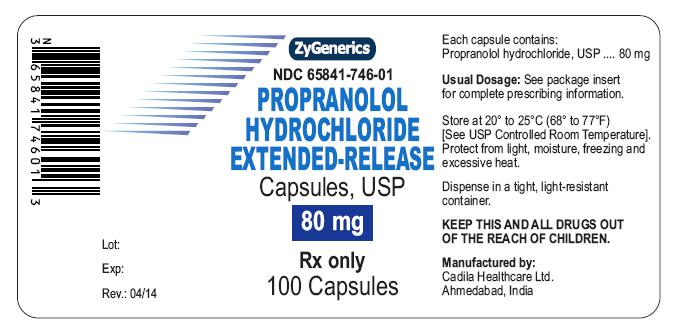
NDC 65841-746-01 in bottle of 100 Capsules
Propranolol Hydrochloride Extended-release Capsules USP, 80 mg
Rx only
100 CAPSULES
NDC 65841-747-01 in bottle of 100 Capsules
Propranolol Hydrochloride Extended-release Capsules USP, 120 mg
Rx only
100 CAPSULES

NDC 65841-748-01 in bottle of 100 Capsules
Propranolol Hydrochloride Extended-release Capsules USP, 160 mg
Rx only
100 CAPSULES
