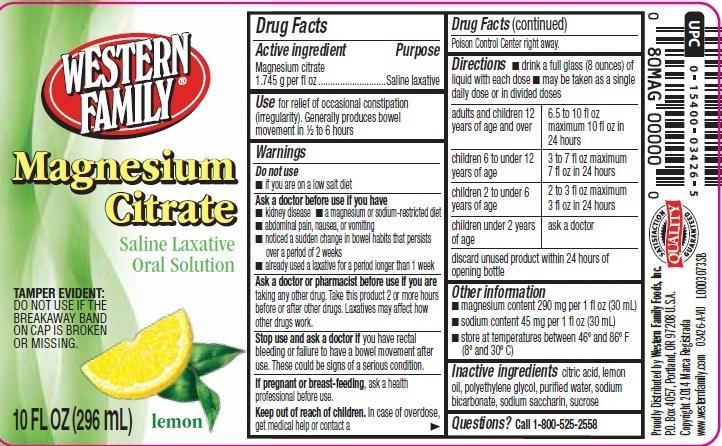
Use
for relief of occasional constipation (irregularity). Generally produces bowel movement in 1/2 to 6 hours
Ask a doctor
before use if you have
- kidney disease
- a magnesium or sodium-restricted diet
- abdominal pain, nausea or vomiting
- noticed a sudden change in bowel habits that persists over a period of 2 weeks
- already use a laxative for a period longer than 1 week
Ask a doctor or pharmacist before use if you are
taking any other drugs. Take this product 2 or more hours before or after other drugs. Laxatives may affect how other drugs work.
Stop Use and ask a doctor if
you have rectal bleeding or failure to have a bowel moverment after use. These could be signs of a serious condition.
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- drink a full glass (8 ounces) of liquid with each dose
- may be taken as a single daily dose or in divided doses
adults and children 12 years of age and over - 6.5 to 10 fl oz maximum 10 fl oz in 24 hours
children 6 to under 12 years of age - 3 to 7 fl oz maximum 7 fl oz in 24 hours
children 2 to under 6 years of age - 2 to 3 fl oz maximum 3 fl oz in 24 hours
children under 2 years of age - ask a doctor
discard unused product within 24 hours of opening bottle
Other information
Other information
- magnesium content 290 mg per 1 fl oz (30 mL)
- sodium content 45 mg per 1 fl oz (30 mL)
- store at temperatures between 46º and 86º F (8º and 30º C)
Inactive ingredients
citric acid, lemon oil, polyethylene glycol, purified water, sodium bicarbonate, sodium saccharin, sucrose