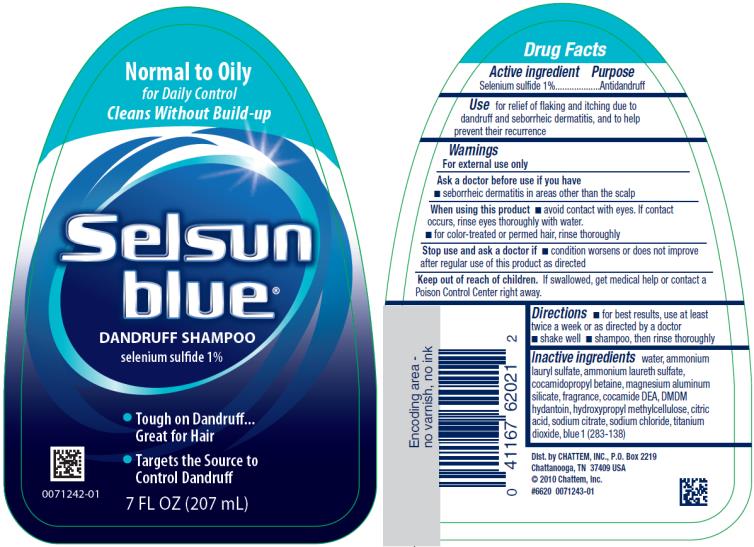
Warnings
For external use only
When using this product
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Directions
- Shake well.
- Wet hair, massage onto scalp, rinse. Repeat if desired.
- For best results, use at least twice a week or as directed by a doctor.
Inactive ingredients
water, ammonium lauryl sulfate, ammonium laureth sulfate, cocamidopropyl betaine, magnesium aluminum silicate, fragrance, cocamide DEA, DMDM hydantoin, hydroxypropyl methylcellulose, citric acid, sodium citrate, sodium chloride, titanium dioxide, blue 1 (283-138)
Drug Facts
Selsun Blue Moisturizing
Warnings
For external use only
When using this product
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Directions
- Shake well.
- Wet hair, massage onto scalp, rinse. Repeat if desired.
- For best results, use at least twice a week or as directed by a doctor.
Inactive ingredients
water, ammonium lauryl sulfate, distearyl phthalic acid amide, ammonium laureth sulfate, sodium chloride, cocamide DEA, dimethicone, aloe barbadensis leaf juice, hydroxypropyl methylcellulose, sodium isostearoyl lactylate, DMDM hydantoin, fragrance, citric acid, sodium citrate, titanium dioxide, blue 1 (283-134)
Drug Facts
Selsun Blue 2-in-1
Warnings
For external use only
When using this product
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Directions
- Shake well.
- Wet hair, massage onto scalp, rinse. Repeat if desired.
- For best results, use at least twice a week or as directed by a doctor.
Inactive ingredients
water, ammonium lauryl sulfate, distearyl phthalic acid amide, ammonium laureth sulfate, sodium chloride, cocamide DEA, dimethicone, hydroxypropyl methylcellulose, DMDM hydantoin, fragrance, citric acid, sodium citrate (283-133)
Drug Facts
Selsun Blue Medicated
Warnings
For external use only
When using this product
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Directions
- Shake well.
- Wet hair, massage onto scalp, rinse. Repeat if desired.
- For best results, use at least twice a week or as directed by a doctor.
Inactive ingredients
water, ammonium lauryl sulfate, TEA lauryl sulfate, ammonium laureth sulfate, cocamidopropyl betaine, magnesium aluminum silicate, fragrance, menthol, cocamide DEA, DMDM hydantoin, citric acid, hydroxypropyl methylcellulose, sodium citrate, sodium chloride, blue 1, red 33 (283-139)
PRINCIPAL DISPLAY PANEL
Normal to Oily
for Daily Control
Cleans Without Build-up
Selsun blue®
DANDRUFF SHAMPOO
selenium sulfide 1%
Tough on Dandruff…Great for Hair
Targets the Source to Control Dandruff
7 FL OZ (207 mL)
PRINCIPAL DISPLAY PANEL
+
MAXIMUM
STRENGTH
Selsun
blue®
Antidandruff Shampoo/
Selenium sulfide 1%
MOISTURIZING
11 FL OZ (325 mL)





