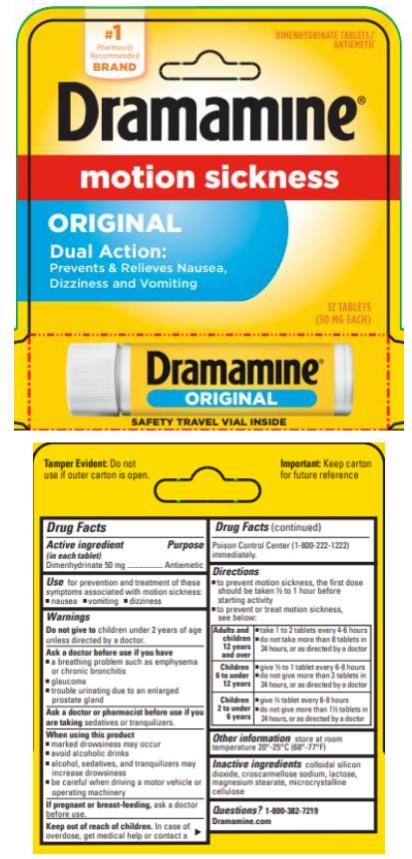
Active ingredient
(in each tablet)
Dimenhydrinate 50 mg
Use
for prevention and treatment of these symptoms associated with motion sickness:
- nausea
- vomiting
- dizziness
Warnings
Do not give to
children under 2 years of age unless directed by a doctor.
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers.
When using this product
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase dizziness
- be careful when driving a motor vehicle or operating machinery
If pregnant or breast-feeding,
ask a doctor before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) immediately.
Directions
- to prevent motion sickness, the first dose should be taken ½ to 1 hour before starting activity
- to prevent or treat motion sickness, see below:
Adults and
children 12
years and over |
- take 1 to 2 tables every 4-6 hours
- do not take more than 8 tablets in
24 hours, or as directed by a doctor
|
Children 6 to
under 12 years |
- give ½ to 1 tablet every 6-8 hours
- do not give more than 3 tablets in
24 hours, or as directed by a doctor
|
Children 2 to
under 6 years |
- give ½ tablet every 6-8 hours
- do not give more than 1 ½ tablets
in 24 hours, or as directed by a doctor
|
Other information
- store at room temperature 20-25ºC (68-77ºF)
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, lactose, magnesium stearate, microcrystalline cellulose
Questions or comments?
Call 1-800-382-7219
Dramamine.com
PRINCIPAL DISPLAY PANEL
Dramamine®
motion sickness
Original
Dimenhydrinate tablets/antiemetic
12 tablets (50 mg each)
Medtech Products Inc.
