Warnings
- do not give to children under 12 years of age
- do not use with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Directions
- adults and children 12 years of age and over: 1 softgel (50 mg) at bedtime if needed, or as directed by a doctor.
Other information
- store at room temperature 15°-30°C (59°-86°F) and avoid excessive heat
- do not use if imprinted safety seal under cap is broken or missing
Inactive ingredients
FD&C blue #1, gelatin, glycerin, polyethylene glycol, purified water, sorbitol special and white edible ink.
Manufactured by:
Humanwell PuraCap Pharmaceutical (Wuhan) Ltd.
Wuhan, Hubei
430206, China
PRINCIPAL DISPLAY PANEL - Shipping Label
DIPHENHYDRAMINE HYDROCHLORIDE CAPSULES, 50 mg
Quantity : 12000 Capsules
NDC. No : 53345-005-01
IMPORTANT:
Inspect immediate upon receipt.
This is a bulk shipment intended for further processing only.
Protect from heat, humidity, and light. Do not refrigerate.
CAUTION : "FOR FURTHER MANUFACTURING, PROCESSING OR REPACKING"


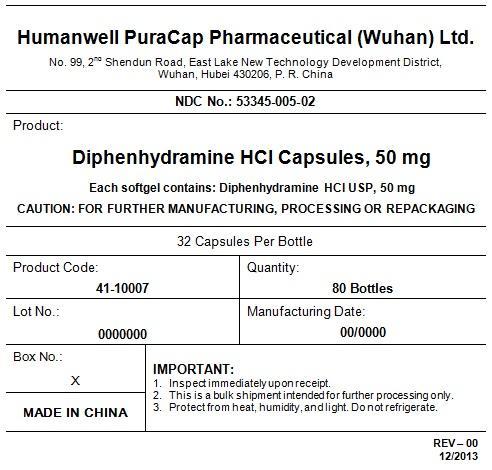
PRINCIPAL DISPLAY PANEL - Shipping Label
DIPHENHYDRAMINE HYDROCHLORIDE CAPSULES, 50 mg
Quantity : 80 Bottles
NDC. No : 53345-005-02
IMPORTANT:
Inspect immediate upon receipt.
This is a bulk shipment intended for further processing only.
Protect from heat, humidity, and light. Do not refrigerate.
CAUTION : "FOR FURTHER MANUFACTURING, PROCESSING OR REPACKING"