COLGATE- sodium fluoride paste
Buzz Export Services Pty., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
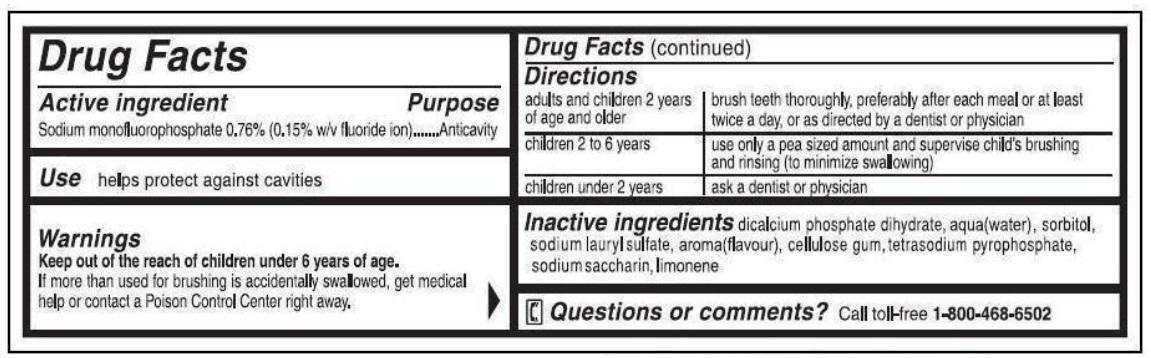
Active Ingredient
Sodium monofluorophosphate 0.76% (0.15% w/v fluoride ion)
Use
helps protect against cavities
Keep out of reach of children under 6 years of age.
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
adults and children 2 years of age and older: brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician
children 2 to 6 years: use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing)
children under 2 years: ask a dentist or physician
Inactive Ingredients
dicalcium phosphate dihydrate, water, glycerin, sodium lauryl sulfate, cellulose gum, flavor, tetrasodium pyrophosphate, sodium saccharin
Questions?
1-800-468-6502
Dist. by: COLGATE-PALMOLIVE COMPANY New York, NY 10022
DRUG FACTS PANEL INSERT
DENTAL KIT - COLGATE TOOTHPASTE 5g Labels
TUBE LABELING:

KIT PACKAGING:

DENTAL KIT - COLGATE TOOTHPASTE 10g
TUBE LABELING:

KIT PACKAGING:

Buzz Export Services Pty., Ltd.




