FULL PRESCRIBING INFORMATION
1. INDICATIONS AND USAGE
PHOTREXA® VISCOUS and PHOTREXA® are indicated for use in corneal collagen cross-linking in combination with the KXL™ System for the treatment of
2. DOSAGE AND ADMINISTRATION
Using topical anesthesia, debride the epithelium to a diameter of approximately 9 mm using standard aseptic technique. Post epithelial debridement, instill 1 drop of PHOTREXA VISCOUS topically on the eye every 2 minutes for 30 minutes.
At the end of the 30 minute soaking period, examine the eye under the slit lamp for the presence of a yellow flare in the anterior chamber. If the yellow flare is not detected, instill 1 drop of PHOTREXA VISCOUS every 2 minutes for an additional 2 to 3 drops and recheck for the presence of a yellow flare. This process can be repeated as necessary.
Once the yellow flare is observed, perform ultrasound pachymetry. If corneal thickness is less than 400 microns, instill 2 drops of PHOTREXA every 5 to 10 seconds until the corneal thickness increases to at least 400 microns. Irradiation should not be performed unless this 400 micron threshold is met and the yellow flare is seen.
Irradiate the eye for 30 continuous minutes at 3mW/cm2 at a wavelength of 365 nm, centered over the cornea, using the KXL System as per the instructions in the KXL manual. During irradiation, continue topical instillation of PHOTREXA VISCOUS onto the eye every 2 minutes for the 30 minute irradiation period.
For topical ophthalmic use. Do not inject.
Single use PHOTREXA VISCOUS and PHOTREXA only. Discard syringe(s) after use.
PHOTREXA VISCOUS and PHOTREXA are for use with the KXL System only.
PLEASE REFER TO THE KXL OPERATOR’S MANUAL FOR SPECIFIC DEVICE INSTRUCTIONS.
3. DOSAGE FORMS AND STRENGTHS
5. WARNINGS AND PRECAUTIONS
Ulcerative keratitis can occur. Monitor for resolution of epithelial defects. [See Adverse Reactions (6)].
6. ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
Ulcerative keratitis [Warnings and Precautions (5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of the corneal collagen cross-linking procedure was evaluated in 3 randomized, parallel-group, open-label, sham-controlled trials; patients were followed up for 12 months. Study 1 enrolled patients with progressive keratoconus or corneal ectasia following refractive surgery. Study 2 enrolled only patients with progressive keratoconus, and Study 3 enrolled only patients with corneal ectasia following refractive surgery. In each study, only one eye of each patient was designated as the study eye. Study eyes were randomized to receive one of the two study treatments (CXL or sham) at the baseline visit and were followed up at Day 1, Week 1, and Months 1, 3, 6, and 12. At Month 3 or later, sham study eyes and non-study eyes had the option of receiving CXL treatment, and were followed-up for 12 months from the time of receiving CXL treatment. Each CXL treated eye received a single course of CXL treatment only.
Safety data were obtained from: 193 randomized CXL study eyes (102 keratoconus, 91 corneal ectasia), 191 control eyes, and 319 nonrandomized CXL non-study eyes (191 keratoconus, 128 corneal ectasia). Overall, 512 eyes (293 keratoconus, 219 corneal ectasia) in 364 patients received CXL treatment.
In progressive keratoconus patients, the most common ocular adverse reactions in any CXL-treated eye were corneal opacity (haze), punctate keratitis, corneal striae, corneal epithelium defect, eye pain, reduced visual acuity, and blurred vision (Table 1).
In corneal ectasia patients, the most common ocular adverse reactions were corneal opacity (haze), corneal epithelium defect, corneal striae, dry eye, eye pain, punctate keratitis, photophobia, reduced visual acuity, and blurred vision. These events are expected sequelae following epithelial corneal debridement and occurred at a higher incidence than observed in control patients, who did not undergo debridement or exposure to UVA light (Table 1).
Adverse events reported in non-study, non-randomized CXL treated were similar in terms of preferred terms and frequency to those seen in randomized study eyes.
The majority of adverse events reported resolved during the first month, while events such as corneal epithelium defect, corneal striae, punctate keratitis, photophobia, dry eye and eye pain, and decreased visual acuity took up to 6 months to resolve and corneal opacity or haze took up to 12 months to resolve. In 1-2% of patients, corneal epithelium defect, corneal edema, corneal opacity and corneal scar continued to be observed at 12 months. In 6% of corneal ectasia patients, corneal opacity continued to be observed at 12 months.
| 1) Results are presented as the number (%) of patients with an event from baseline to Month 3. | ||||
| 2) Almost all cases of corneal opacity were reported as haze. | ||||
| Progressive Keratoconus Studies | Corneal Ectasia Studies |
|||
| Preferred Term | CXL Group (N=102)1 | Control Group (N=103)1 | CXL Group (N=91)1 | Control Group (N=88)1 |
| Anterior chamber cell | 2 (2) | 0 | 2 (2) | 1 (1) |
| Anterior chamber flare | 4 (4) | 0 | 5 (6) | 2 (2) |
| Asthenopia | 1 (1) | 1 (1) | 2 (2) | 0 |
| Blepharitis | 0 | 0 | 0 | 1 (1) |
| Corneal disorder | 3 (3) | 1 (1) | 3 (3) | 0 |
| Corneal epithelium defect | 24 (24) | 1 (1) | 26 (28) | 3 (3) |
| Corneal oedema | 3 (3) | 0 | 3 (3) | 0 |
| Corneal opacity2 | 65 (64) | 9 (9) | 65 (71) | 8 (9) |
| Corneal striae | 24 (24) | 12 (12) | 8 (9) | 6 (7) |
| Corneal thinning | 1 (1) | 2 (2) | 0 | 0 |
| Diplopia | 2 (2) | 1 (1) | 1 (1) | 0 |
| Dry eye | 6 (6) | 2 (2) | 13 (14) | 4 (5) |
| Eye complication associated with device | 2 (2) | 0 | 1 (1) | 0 |
| Eye discharge | 2 (2) | 1 (1) | 0 | 0 |
| Eye oedema | 7 (7) | 0 | 0 | 0 |
| Eye pain | 17 (17) | 3 (3) | 24 (26) | 0 |
| Eye pruritus | 2 (2) | 0 | 0 | 0 |
| Eyelid oedema | 5 (5) | 0 | 5 (6) | 1 (1) |
| Foreign body sensation in eyes | 15 (15) | 1 (1) | 13 (14) | 2 (2) |
| Glare | 4 (4) | 1 (1) | 2 (2) | 0 |
| Halo vision | 1 (1) | 0 | 2 (2) | 0 |
| Keratitis | 1 (1) | 0 | 3 (3) | 0 |
| Lacrimation increased | 5 (5) | 0 | 9 (10) | 1 (1) |
| Meibomian gland dysfunction | 1 (1) | 1 (1) | 3 (3) | 2 (2) |
| Ocular discomfort | 0 | 0 | 8 (9) | 0 |
| Ocular hyperaemia | 14 (14) | 2 (2) | 7 (8) | 4 (5) |
| Photophobia | 11 (11) | 0 | 17 (19) | 0 |
| Punctate keratitis | 25 (25) | 8 (8) | 18 (20) | 3 (3) |
| Vision blurred | 16 (16) | 2 (2) | 15 (17) | 4 (5) |
| Visual acuity reduced | 10 (10) | 9 (9) | 10 (11) | 1 (1) |
| Visual impairment | 3 (3) | 2 (2) | 4 (4) | 1 (1) |
| Vitreous detachment | 2 (2) | 0 | 0 | 0 |
Headache was reported in between 4 to 8% of treated patients.
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
Risk Summary
Animal development and reproduction studies have not been conducted with the PHOTREXA® VISCOUS/PHOTREXA®/KXL® System. Since it is not known whether the corneal collagen cross-linking procedure can cause fetal harm or affect reproduction capacity, it should not be performed on pregnant women.
8.2. Lactation
Risk Summary
There are no data on the presence of PHOTREXA VISCOUS or PHOTREXA in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for the PHOTREXA/KXL corneal collagen cross-linking procedure and any potential adverse effects on the breastfed child from the PHOTREXA/KXL corneal collagen cross-linking procedure or from the underlying maternal condition.
11. DESCRIPTION
PHOTREXA VISCOUS (riboflavin 5’-phosphate in 20% dextran ophthalmic solution) 0.146% and PHOTREXA (riboflavin 5’-phosphate ophthalmic solution) 0.146% are intended for topical ophthalmic administration as part of corneal collagen cross-linking with the KXL System.
PHOTREXA VISCOUS and PHOTREXA are supplied as:
- PHOTREXA VISCOUS in a 3 mL glass syringe containing sterile 1.56 mg/mL riboflavin 5’-phosphate in 20% dextran ophthalmic solution for topical administration.
- PHOTREXA in a 3 mL glass syringe containing sterile 1.46 mg/mL riboflavin 5’-phosphate ophthalmic solution for topical administration.
PHOTREXA VISCOUS (riboflavin 5’-phosphate in 20% dextran ophthalmic solution) 0.146% is a yellow sterile buffered viscous solution containing 1.56 mg/mL riboflavin 5’-phosphate and 20% dextran 500. The pH of the solution is approximately 7.1 and the osmolality is 284-368 mOsm/kg. Each 1 mL of solution contains 1.64 mg of riboflavin 5’-phosphate sodium (equivalent to 1.284 mg riboflavin). Riboflavin 5’-phosphate sodium USP is a mixture of the sodium salts of riboflavin, riboflavin monophosphates, and riboflavin diphosphates. The inactive ingredients are dibasic sodium phosphate, dextran, monobasic sodium phosphate, sodium chloride, and water for injection.
PHOTREXA (riboflavin 5’-phosphate ophthalmic solution) 0.146% is a yellow sterile buffered solution containing 1.46 mg/mL riboflavin 5’-phosphate. The pH of the solution is approximately 7.1 and the osmolality is 157-181 mOsm/kg. Each 1 mL of solution contains 1.53 mg of riboflavin 5’-phosphate sodium (equivalent to 1.20 mg riboflavin). Riboflavin 5’-phosphate sodium USP is a mixture of the sodium salts of riboflavin, riboflavin monophosphates, and riboflavin diphosphates. The inactive ingredients are dibasic sodium phosphate, monobasic sodium phosphate, sodium chloride, and water for injection.
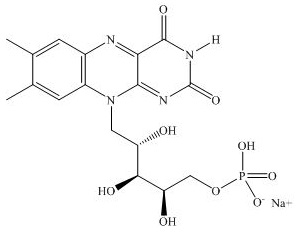
The chemical formula for riboflavin 5’-phosphate sodium (Vitamin B2) is C17H20N4NaO9P with a molecular mass of 478.33 g/mol.
Please refer to the KXL System Operator’s Manual for a specific device description and instructions.
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
Riboflavin 5’-phosphate sodium (Vitamin B2) is the precursor of two coenzymes, flavin adenine dinucleotide and flavin mononucleotide, which catalyze oxidation/reduction reactions involved in a number of metabolic pathways.
Under the conditions used for corneal collagen cross-linking, riboflavin 5‘-phosphate functions as a photoenhancer and generates singlet oxygen which is responsible for the cross-linking.
13. NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies have not been conducted to determine the carcinogenic potential of photoexcited riboflavin. Photoexcited riboflavin has been shown to be genotoxic in the Ames Salmonella reverse mutation assay and in the SOS/umu test system.
The genotoxicity of riboflavin, in the absence of photoexcitation has been examined in vitro in bacterial reverse mutation assays, sister chromatid exchange assay, chromosomal aberration assays and in vivo in a mouse micronucleus study. The overall weight of evidence indicates that riboflavin, in the absence of photoexcitation, is not genotoxic.
Animal studies to determine the effects of the PHOTREXA/KXL corneal collagen cross-linking procedure on fertility were not conducted.
14. CLINICAL STUDIES
Three prospective, randomized, parallel-group, open-label, sham-controlled trials were conducted to evaluate the safety and effectiveness of riboflavin ophthalmic solution/UVA irradiation for performing corneal collagen cross-linking. These trials were sham-controlled for the first 3 months and had a total duration of 12 months for safety and efficacy evaluations. Study 1 enrolled 58 patients with progressive keratoconus and 49 patients with corneal ectasia following refractive surgery. Study 2 enrolled 147 patients with progressive keratoconus, and Study 3 enrolled 130 patients with corneal ectasia following refractive surgery. In each study, patients had one eye designated as the study eye and were randomized to receive one of two study treatments (CXL or sham) in their study eye at the baseline visit. The patients were evaluated at Day 1, Week 1, and Months 1, 3, 6, and 12. At Month 3 or later, patients had the option of receiving CXL treatment in both the sham study eyes and non-study eyes and were followed-up for 12 months from the time of receiving CXL treatment.
Approximately 56% and 89% of the sham study eyes in patients with progressive keratoconus received CXL treatment by Month 3 and Month 6, respectively. The average age of keratoconus patients was 33 years. The average baseline Kmax value was 61 diopters. For corneal ectasia patients in Study 1 and Study 3, approximately 60% and 90% of the sham study eyes received CXL treatment by Month 3 and Month 6, respectively. The average age of corneal ectasia patients was 43 years and the average baseline Kmax was 55 diopters. A majority (93%) of the corneal ectasia patients had LASIK only, 5 (3%) patients had photorefractive keratectomy (PRK) only, and 8 (4%) patients had both LASIK and PRK.
In each study, the maximum corneal curvature (Kmax) was assessed at baseline, Months 1, 3, and 12. The CXL-treated eyes showed increasing improvement in Kmax from Month 3 through Month 12 (Figure 1). Progressive keratoconus patients had an average Kmax reduction of 1.4 diopters in Study 1 and 1.7 diopters in Study 2 at Month 12 in the CXL-treated eyes while the sham eyes had an average increase of 0.5 diopter in Study 1 and 0.6 diopter in Study 2 at Month 12; the difference (95% CI) between the CXL and sham groups in the mean change from baseline Kmax were -1.9 (-3.4, -0.3) diopters in Study 1 and -2.3 (-3.5, -1.0) diopters in Study 2.
For corneal ectasia patients, at Month 12, the CXL-treated eyes had an average Kmax reduction of 1.0 diopter in Study 1 and 0.5 diopter in Study 3 while the sham eyes had an average increase of 1.0 diopter in Study 1 and 0.5 diopter in Study 3; the treatment difference between the CXL and sham groups was: -2.0 (-3.0, -1.1) diopters in Study 1 and -1.1 (-1.9, -0.3) diopters in Study 3.
Figure 1: Mean (SD) (Diopter) Baseline Kmax and Change from Baseline Kmax
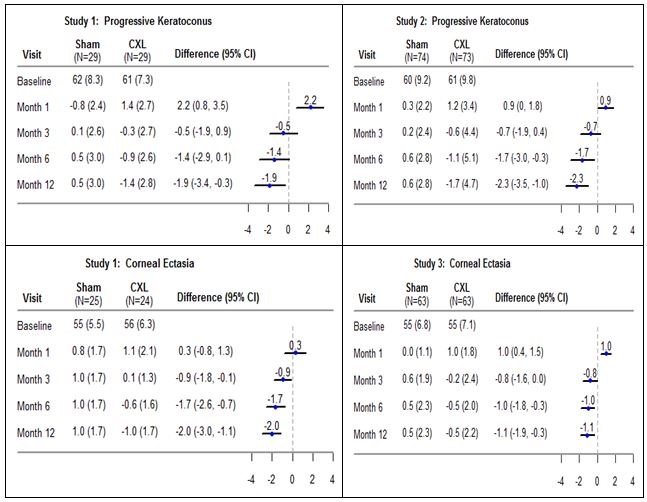
Post-baseline missing data were imputed using last available Kmax value. For the sham study eyes that received CXL treatment after baseline, the last Kmax measurement recorded prior to receiving CXL treatment was used in the analysis for later time points. In Study 3, four patients in the CXL group had missing baseline Kmax value and were excluded from the analysis.
16. HOW SUPPLIED/STORAGE AND HANDLING
Single-use foil pouches of PHOTREXA® VISCOUS and PHOTREXA® are provided in a kit of two (2): one (1) PHOTREXA® VISCOUS and one (1) PHOTREXA® (NDC- 25357-025-03).
Each foil pouch contains a 3 mL glass syringe of PHOTREXA® VISCOUS or PHOTREXA® contained within a Tyvek® pouch.
Kits should be stored at 2°-8°C (36°-46°F). Care should be taken to minimize exposure of the syringe to light once removed from its protective packaging. Discard syringe after use.
For topical ophthalmic use.
PHOTREXA® VISCOUS and PHOTREXA® should be used with the KXL System only.
17. PATIENT COUNSELING INFORMATION
- Patients should be advised not to rub their eyes for the first five days after their procedure.
- Patients may be sensitive to light and have a foreign body sensation. Patients should be advised that there may be discomfort in the treated eye and that sunglasses may help with light sensitivity.
- If patients experience severe pain in the eye or any sudden decrease in their vision, they should be advised to contact their physician immediately.
- If the bandage contact lens that was placed on the patient’s eye on the day of treatment falls out or becomes dislodged, the patient should be advised not to replace it and to contact their physician immediately.
PHOTREXA® VISCOUS (riboflavin 5’-phosphate in 20% dextran ophthalmic solution) 0.146%, PHOTREXA® (riboflavin 5’-phosphate ophthalmic solution) 0.146% and the KXL® System are marketed by: Avedro, a Glaukos company, 30 North Avenue, Burlington, MA 01803.
Version:
Issued October 2022
ML-00043 REV2






