EYEWASH- water solution
Henry Schein, Inc.
----------
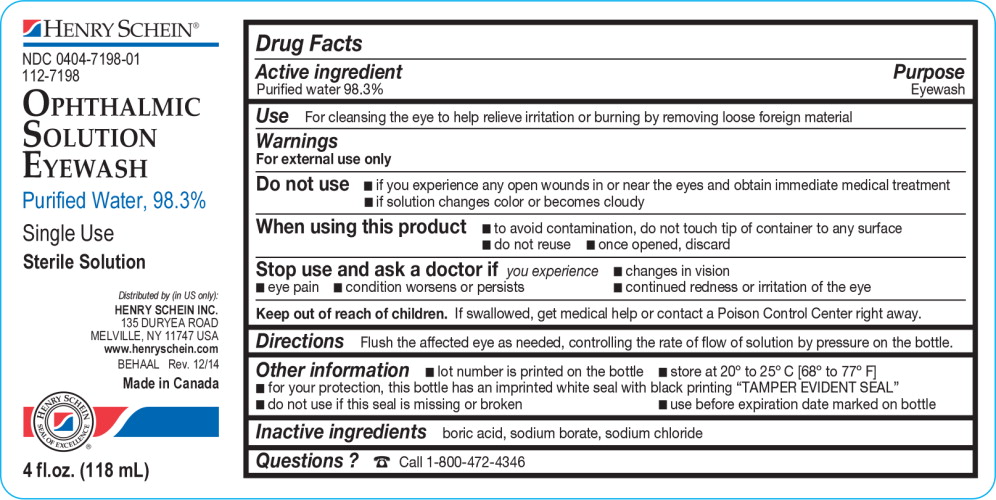
Henry Schein Eyewash
Warnings
For external use only
Do not use
- if you experience any open wounds in or near the eyes and obtain immediate medical treatment
- if solution changes color or becomes cloudy
When using this product
- to avoid contamination, do not touch tip of container to any surface
- do not reuse
- once opened, discard
Directions
Flush the affected eye as needed, controlling the rate of flow of solution by pressure on the bottle.
Other information
- lot number is printed on the bottle
- store at 20º to 25º C [68º to 77º F]
- for your protection, this bottle has an imprinted white seal with black printing “TAMPER EVIDENT SEAL”
- do not use if this seal is missing or broken
- use before expiration date marked on bottle
Principal Display Panel Text for Container Label:
HENRY SCHEIN logo
NDC 0404-7198-01
112-7198
Ophthalmic
Solution
Eyewash
Purified Water, 98.3%
Single Use
Sterile Solution
Distributed by (in US only):
HENRY SCHEIN INC.
135 DURYEA ROAD
MELVILLE, NY 11747 USA
www.henryschein.com
BEHAAL Rev. 12/14
Made in Canada
4 fl.oz. (118 mL)
| EYEWASH
water solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Henry Schein, Inc. (012430880) |
| Registrant - Akorn Operating Company LLC (117693100) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Niagara Pharmaceuticals, Inc. | 205477792 | manufacture(0404-7198) | |
Revised: 11/2023
Document Id: ff03f954-dfef-4395-b13b-2b9fe6b4fff0
Set id: f7c5887c-05a3-4831-82eb-d8aea6706571
Version: 5
Effective Time: 20231127
Henry Schein, Inc.