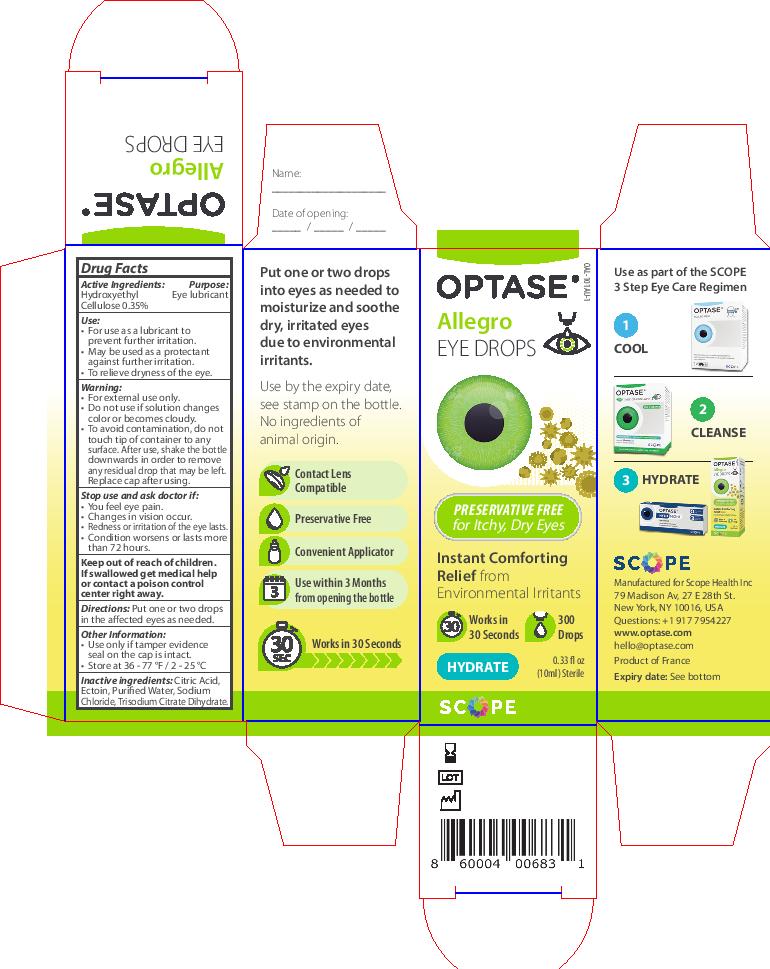
Keep out of reach of children.
If swallowed get medical
help or contact a poison
control center right away.
Inactive ingredients: Citric Acid,
Ectoin, Purified Water, Sodium
Chloride, Trisodium Citrate Dihydrate.
Warning
• For external use only.
• If solution changes color or
becomes cloudy, do not use.
To avoid contamination, do
not touch tip to any surface.
After use, shake the bottle
downwards in order to remove
any residual drop that may be left.
Replace cap after using.
Stop use and ask doctor if:
Stop use and ask doctor if
• You feel eye pain.
• Changes in vision occur.
• Redness or irritation of the eye lasts.
• Condition worsens or lasts
more than 72 hours