INDICATIONS:
For staining the anterior segment of the eye when fitting contact lenses, in disclosing corneal injury and in applanation tonometry.
DIRECTIONS FOR USE:
To insure full fluorescence and patient comfort, the BioGlo impregnated tip should be moistened before application. One or two drops of sterile irrigating or saline solution should be used for this purpose. Touch conjunctiva or fornix as required with moistened tip. It is recommended that the patient blink several times after application.
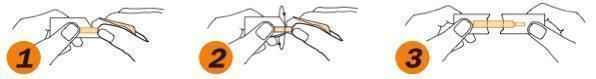
- Grasp tabs between thumbs & index fingers
- Gently pull tabs apart
- Remove strip taking care to touch only the white portion of the strip
NOTE:
For external use only. Contents may not be sterile if individual strips have been damaged or previously opened.


