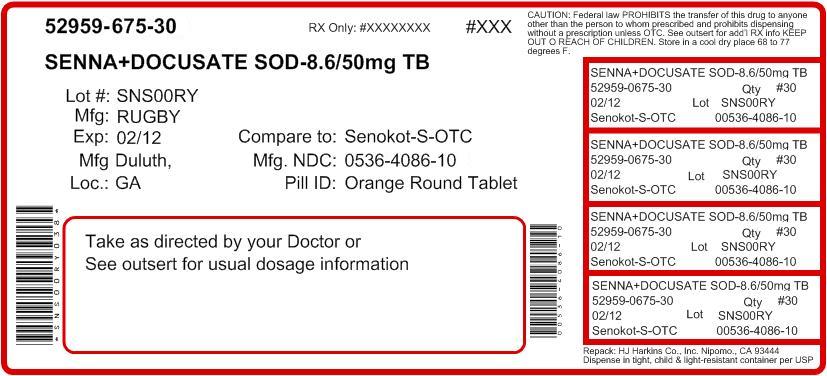
Ask a doctor or pharmacist berfore use if you are taking any other drug. Laxatives may affect how other drugs work.
Do not use if your are taking mineral oil; for longer than one week; when abdominal pain, nausea or vomiting are present
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if: you have rectal bleeding; you fail to have a bowel movement after use of this product. These may indicate a serious condition.
Inactive ingredients
carnauba wax, colloidal silicon dioxide, croscarmellose sodium
D-C yellow #10 aluminum lake, dibasic calcium phosphate
dihydrate, FD-C yellow #6 aluminum lake, hypromellose,
magnesium stearate, microcrystalline cellulose, polyethylene
glycol, sodium benzoate, stearic acide, Titanium dioxide
Directions take preferably at bedtime or as directed by a doctor
If you do not have a comfortable bowel movement by the second day, increase dose by one tablet (do not exceed maxiumum dosage)
Dosage and Administration
Adults and children 12 years and over - 2 tablets once a day - maximum dosage - 4 tablets twice a day
children 6 to under 12 years - 1 tablet once a day maximum dosage - 2 tablets twice a day
children 2 to under 6 years - 1/2 tablet once a day - maximum dosage- 1 tablet twice a day
children under 2 years - ask a doctor
 Enter section text here
Enter section text here