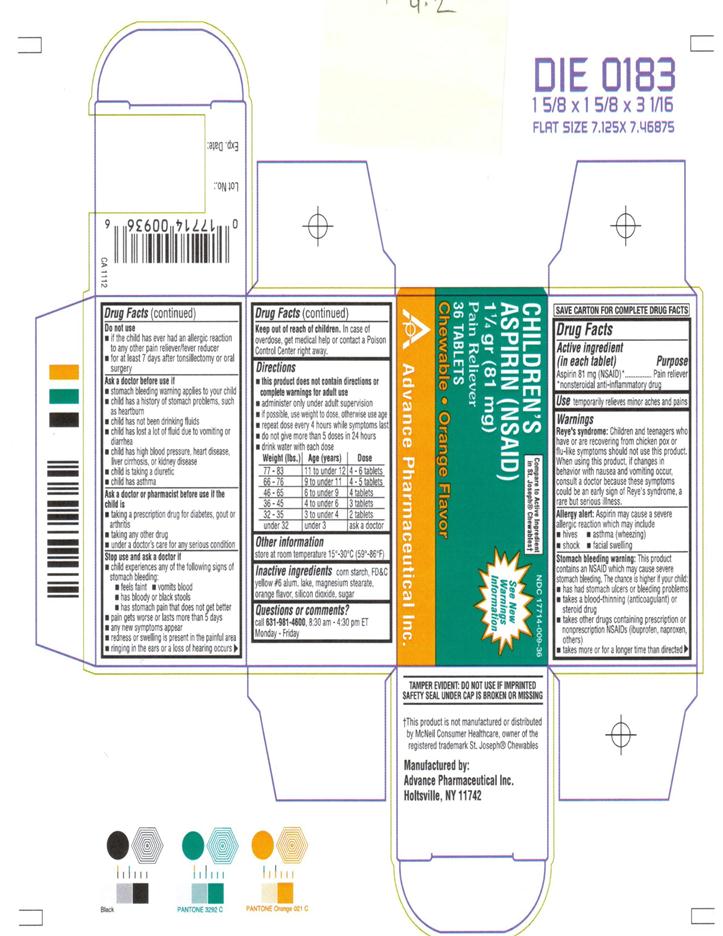
Use
- temporarily relieves minor aches and pains
- for other uses, see your doctor, but do not use for more than 10 days without consulting your doctor because serious side effect may occur.
Warnings
Reye’s Syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early signs of Reye’s syndrome, a rare but serious illness.
Allergy Alert: Aspirin may cause a severe allergic reaction which may include
- hives
- asthma (wheezing)
- shock
- facial swelling
Stomach Bleeding Warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if your child
- has had stomach ulcers or bleeding problems
- take a blood-thinning (anticoagulant) or steroid drug
- takes other drugs containing prescription or nonprescription NSAIDs (ibuprofen, naproxen, others)
- takes more or for a longer time than directed
Do not use
- if the child has ever had an allergic reaction to any other pain reliever / fever reducer
- for at least 7 days after tonsillectomy or oral surgery
Ask a doctor before use if
- stomach bleeding warning applies to your child
- child has a history of stomach problems, such as heartburn
- child has not been drinking fluids
- child has lost a lot of fluid due to vomiting and diarrhea
- child has high blood pressure, heart disease, lever cirrhosis, or kidney disease
- child is taking a diuretic
- child has asthma
Ask a doctor or pharmacist before use if the child is
- taking a prescription drug for diabetes, gout or arthritis
- taking any other drug
- under a doctor’s care for any serious condition
Stop use and ask a doctor if
- child experiences any of the following signs of stomach bleeding:
- feels faint
- vomits blood
- has bloody or black stools
- has stomach pain that does not get better
- pain gets worse or lasts more than 5 days
- any new symptoms appear
- redness or swelling is present in the painful area
- ringing in the ears or a loss of hearing occurs
If pregnant or breast-feeding, ask a health professional before use. It is especially important not to use aspirin during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- this product does not contain directions or complete warnings for adult use
- administer only under adult supervision
- if possible, use weight to dose, otherwise use age
- repeat dose every 4 hours while symptoms last
- do not give more than 5 doses in 24 hours
- drink water with each dose
Weight (lbs.) Age (years) Dose 77 – 83 11 to under 12 4 – 6 tablets 66 – 76 9 to under 11 4 - 5 tablets 46 – 65 6 to under 9 4 tablets 36 – 45 4 to under 6 3 tablets 32 – 35 3 to under 4 2 tablets under 32 under 3 ask a doctor DRINK A FULL GLASS OF WATER WITH EACH DOSE
- adults and children 12 years and older : take 4 to 8 tablets every 4 hours while symptoms last, but not more than 48 tablets in 24 hours.
- Children under 12 years: ask a doctor
Inactive Ingredients
corn starch, FD&C Yellow #6 alum. lake, magnesium stearate, orange flavor, silicon dioxide, sugar