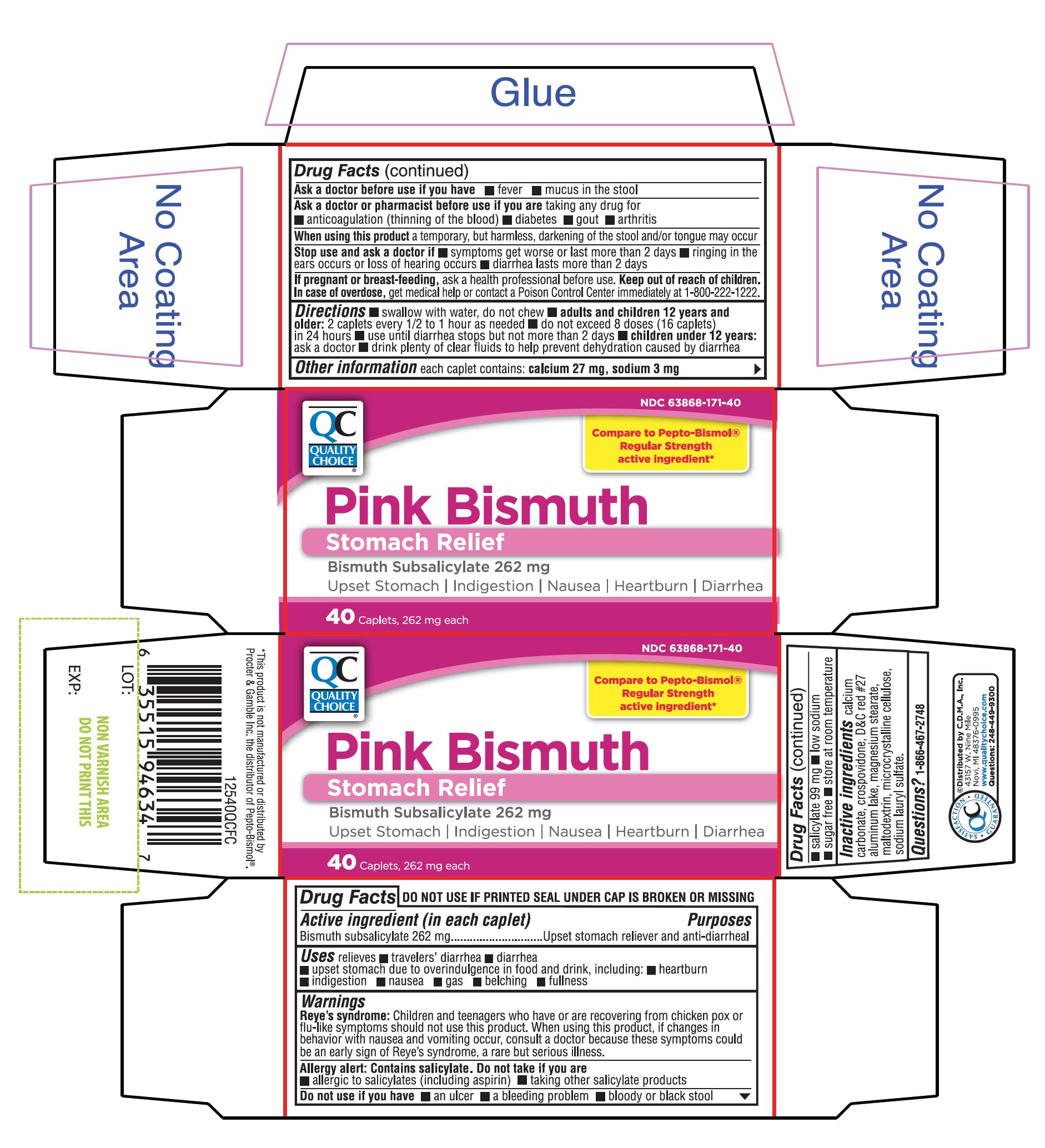
Uses
relieves:
■ travelers’ diarrhea
■ diarrhea
■ upset stomach due to overindulgence in food and drink, including: ■ heartburn ■ indigestion ■ nausea ■ gas ■ belching ■ fullness
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are
- ▪
- allergic to salicylates (including aspirin)
- ▪
- taking other salicylate products.
Ask a doctor or pharmacist before use if you are
taking any drug for
- ▪
- anticoagulation (thinning the blood)
- ▪
- diabetes
- ▪
- gout
- ▪
- arthritis
Directions
- ▪
- swallow with water, do not chew
- ▪
- adults and children 12 years and older: 2 caplets every 1/2 to 1 hour as needed
- ▪
- do not exceed 8 doses (16 caplets) in 24 hours
- ▪
- use until diarrhea stops but not more than 2 days
- ▪
- children under 12 years: ask a doctor
- ▪
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
Other Information
- ▪
- each caplet contains: calcium 27 mg, sodium 3 mg
- ▪
- salicylate 99 mg
- ▪
- low sodium
- ▪
- sugar free
- ▪
- store at room temperature.
NDC# 63868-171-40
Compare to Pepto-Bismol® Regular Strength the active ingredient*
Pink Bismuth
Stomach Relief
Bismuth Subsalicylate 262mg
Upset stomach Indigestion, Nausea, Heartburn, Diarrhea
Package Contains One Bottle
40 Caplets
QC SATISFACTION GUARANTEED
Distributed by C.D.M.A., Inc.
43157 W. Nine Mile
Novi MI 48376-0995
Questions: 248-449-9300
*This product is not manufactured or distributed by Procter & Gamble Inc., the distributor of Pepto-Bismol®.