Fluorescein Sodium Ophthalmic Strips U.S.P. diagnostic agent is for professional use only
Each strip is impregnated with 0.6 mg of fluorescein sodium USP.
INDICATIONS
For staining the anterior segment of the eye in disclosing corneal injury, in applanation tonometry and when fitting contact lenses.
DIRECTIONS FOR USE
To ensure full fluorescence and patient comfort, the GloStrip® impregnated tip should be moistened with one or two drops of sterile, isotonic saline or irrigating solution before application.
Touch conjunctiva or fornix as required with moistened tip. It is recommended that the patient blink several times after application.
INSTRUCTIONS FOR OPENING STERILE GLOSTRIPS®
| 1.Grasp free tab ends of wrapping and slowly pull apart. When the white paper handle becomes visible, remove the GloStrip® from the envelope. | 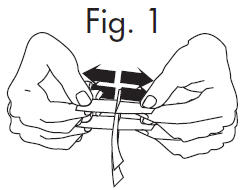 |
An Alternate Method of Opening
| 1.Grasp envelope firmly with two hands as shown in Fig. 2 below. Tear the envelope from both its edges to the strip handle. | 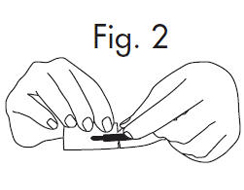 |
| 2. Hold the handle end of the GloStrip® with the left hand and the paper envelope without holding the tip with the right hand. See Fig. 3 | 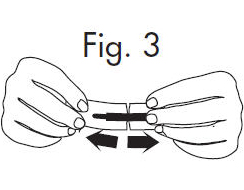 |
| 3. Separate the envelope at the tear, exposing the GloStrip® tip. See Fig. 4 | 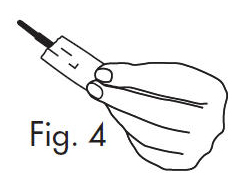 |
