COLDSORE BOMB- menthol ointment
Envisionate PSJ LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
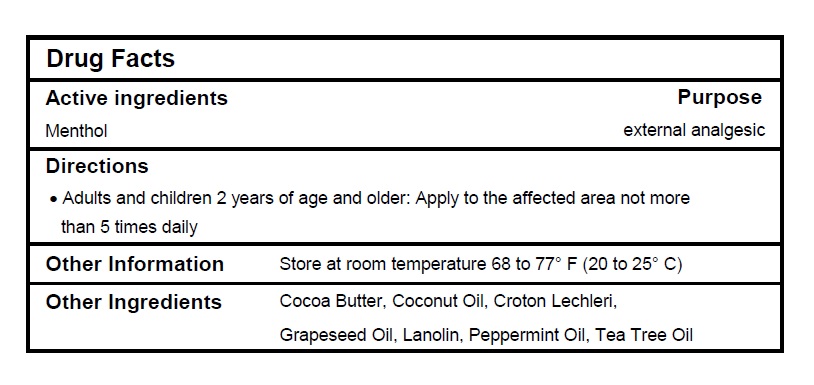
Active ingredients
Menthol 1%
Purpose
External analgesic
Directions
- Adults and children 2 years of age and older: Apply to the affected area not more than 5 times daily.
Other Information
Store at room temperature 68 to 77°F (20 to 25ºC).
Inactive Ingredients
Cocoa Butter, Coconut Oil, Croton Lechleri, Grapeseed Oil, Lanolin, Peppermint Oil, Tea Tree Oil
Packaging