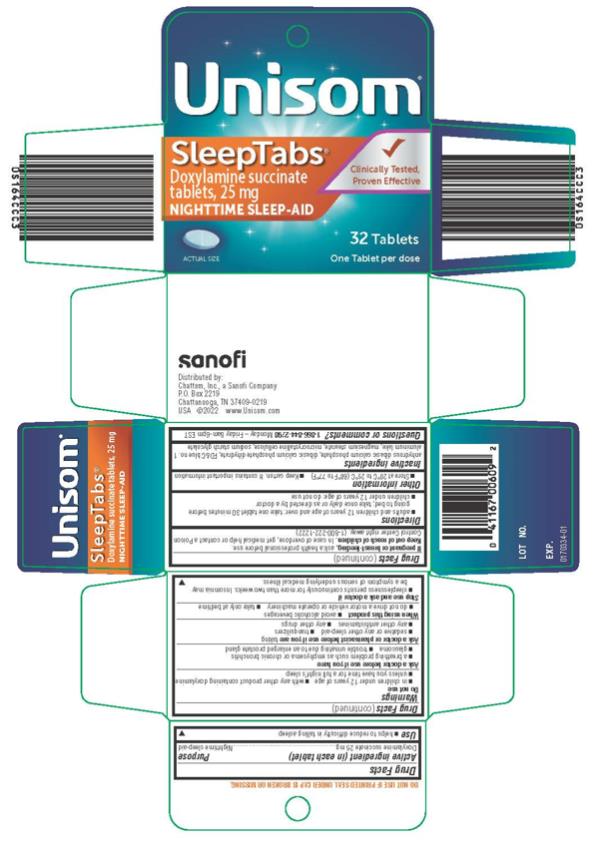
Unisom® SleepTabs®
Doxylamine succinate tablets Nighttime Sleep-Aid
Drug Facts
Active ingredient (in each tablet)
Doxylamine succinate 25 mg
Purpose
Nighttime sleep-aid
Uses
- helps to reduce difficulty in falling asleep
Warnings
Do not use
- in children under 12 years of age
- with any other product containing doxylamine
- unless you have time for a full night’s sleep
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are taking
- sedative or any other sleep-aid
- tranquilizers
- any other antihistamines
- any other drugs
When using this product
- avoid alcoholic beverages
- do not drive a motor vehicle or operate machinery
- take only at bedtime
Stop use and ask a doctor if
- sleeplessness persists continuously for more than two weeks. Insomnia may be a symptom of serious underlying medical illness.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
- adults and children 12 years of age and over: take one tablet 30 minutes before going to bed; take once daily or as directed by a doctor
- children under 12 years of age: do not use
Other information
- Store at 20º to 25ºC (68 º-77 ºF)
- Keep carton. It contains important information
Inactive ingredients
anhydrous dibasic calcium phosphate, dibasic calcium phosphate dihydrate, FD&C blue no. 1 aluminum lake, magnesium stearate, microcrystalline cellulose, sodium starch glycolate
Questions or comments: 1-866-844-2798 Monday- Friday 8am-6pm EST
PRINCIPAL DISPLAY PANEL
Unisom Sleeptabs
Doxylamine succinate
25 mg
Nighttime Sleep-Aid
32 Tablets
Chattem, Inc.
