Keep out of reach of children. In case of overdose get medical help or contact a poison control center right away.
Oral Analgesic. Aspirin is not appropriate for everyone, so be sure to talk to a doctor before starting an Aspirin regimen.
Do not use this product if you are allergic to Aspirin or any other pain/fever product.
If pregnant or breastfeeding contact a doctor before using.
Contact a doctor or pharmacist if you are taking a prescription drug for-Gout, Diabetes or Arthritis.
Contact a doctor right away if: An allergic reaction occurs.
Drink a full glass of water with each dose.
Adults and children 12 years of age and up:Take 4-8 tablets every 4 hours not to exceed 48 tablets in 24hours unless indicated by a doctor.. Children under 12 years of age : Consult a Doctor.
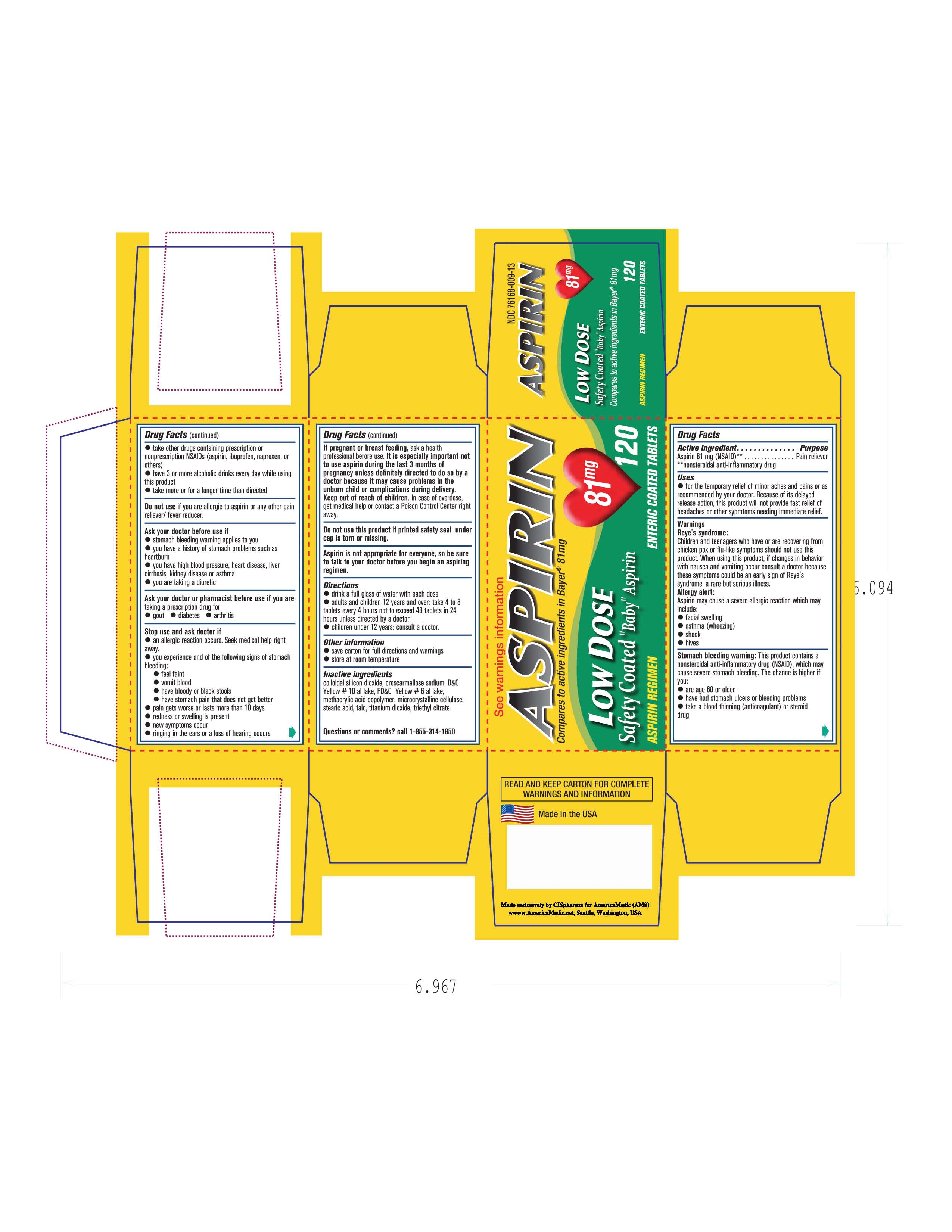 Image of carton
Image of carton