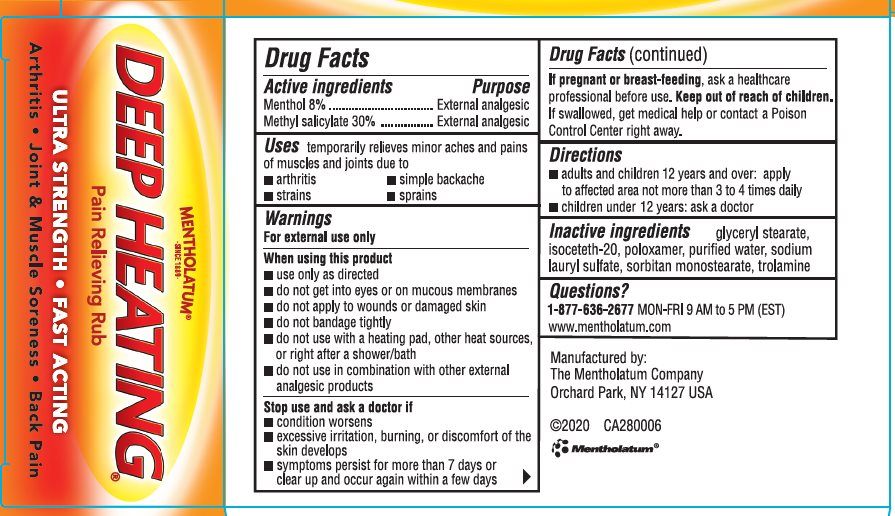
MENTHOLATUM DEEP HEATING RUB
EXTRA STRENGTH- menthol, methyl salicylate cream
The Mentholatum Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients
Menthol 8%
Methyl salicylate 30%
Purpose
Menthol - External analgesic
Methyl salicylate - External analgesic
Uses
temporarily relieves minor aches and pains of muscles and joints due to
- arthritis
- strains
- simple backache
- sprains
Warnings
For external use only
When using this product
- use only as directed
- do not get into eyes or on mucous membranes
- do not apply to wounds or damaged skin
- do not bandage tightly
- do not use with heating pad, other heat sources, or right after a shower/bath
- do not use in combination with other external analgesic products
Stop use and ask a doctor if
- condition worsens
- excessive irritation, burning, or discomfort of the skin develops
- symptoms persist for more than 7 days or clear up and occur again within a few days
If pregnant or breast-feeding, ask a healthcare professional before use.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 years and over: apply to affected area not more than 3 to 4 times daily
- children under 2 years: ask a doctor
Inactive ingredients
glyceryl stearate, isoceteh-20, poloxamer, purified water, sodium lauryl sulfate, sorbitan monostearate, trolamine
Questions?
1-877-636-2677 MON-FRI 9 AM to 5 PM (EST)
www.mentholatum.com
Package/Label Principal Display Panel