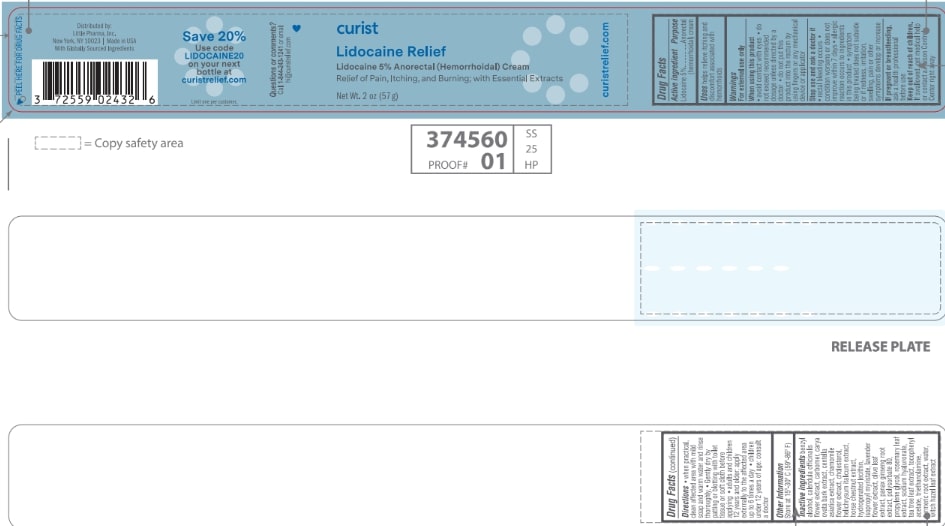
CURIST LIDOCAINE RELIEF- lidocaine 5% cream
Little Pharma, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Lidocaine 5%
Purpose
Anorectal (hemorrhoidal) cream
Uses
helps relieve itching and discomfort associated with hemorrhoids
Warnings
For external use only
When using this product
- avoid contact with eyes
- do not exceed recommended dosage unless directed by a doctor
- do not put this product into the rectum by using fingers or any mechanical device or applicator
Stop use and ask a doctor if
- rectal bleeding occurs
- condition worsens or does not improve within 7 days
- allergic reaction occurs to ingredients in this product
- symptom being treated does not subside or if redness, irritation, swelling, pain or other symptoms develop or increase
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Directions
- when practical, clean affected area with mild soap and warm water and rinse thoroughly
- Gently dry by patting or blotting with toilet tissue or soft cloth before applying
- adults and children 12 years and older: apply externally to the affected area up to 6 times a day
- children under 12 years of age: consult a doctor
Other information
Store at 15°-30° C (59°-86° F)
Inactive ingredients benzyl alcohol, calendula officinalis flower extract, carbomer, carya ovata bark extract, centella asiatica extract, chamomile flower extract, cholesterol, helichrysum italicum extract, horse chestnut extract, hydrogenated lecithin, isopropyl myristate, lavender flower extract, olive leaf extract, panax ginseng root extract, polysorbate 80, propylene glycol, rosemary leaf extract, sodium hyaluronate, tea tree leaf extract, tocopheryl acetate, triethanolamine, turmeric root extract, water, witch hazel leaf extract
curistrelief.com
PEEL HERE FOR DRUG FACTS
Distributed by:
Little Pharma, Inc.
New York, NY 10023 | Made in USA
With Globally Sourced Ingredients
Save 20%
Use code
LIDOCAINE20
on your next bottle at
curistrelief.com
Limit one per customer.
Questions or comments?
Call
1-844-243-1241 or email hi@curistrelief.com
curist
Lidocaine Relief
Lidocaine 5% Anorectal (Hemorrhoidal) Cream
Relief of Pain, Itching and Burning; with Essential Extracts
Net Wt. 2 oz (57 g)
Little Pharma, Inc.
