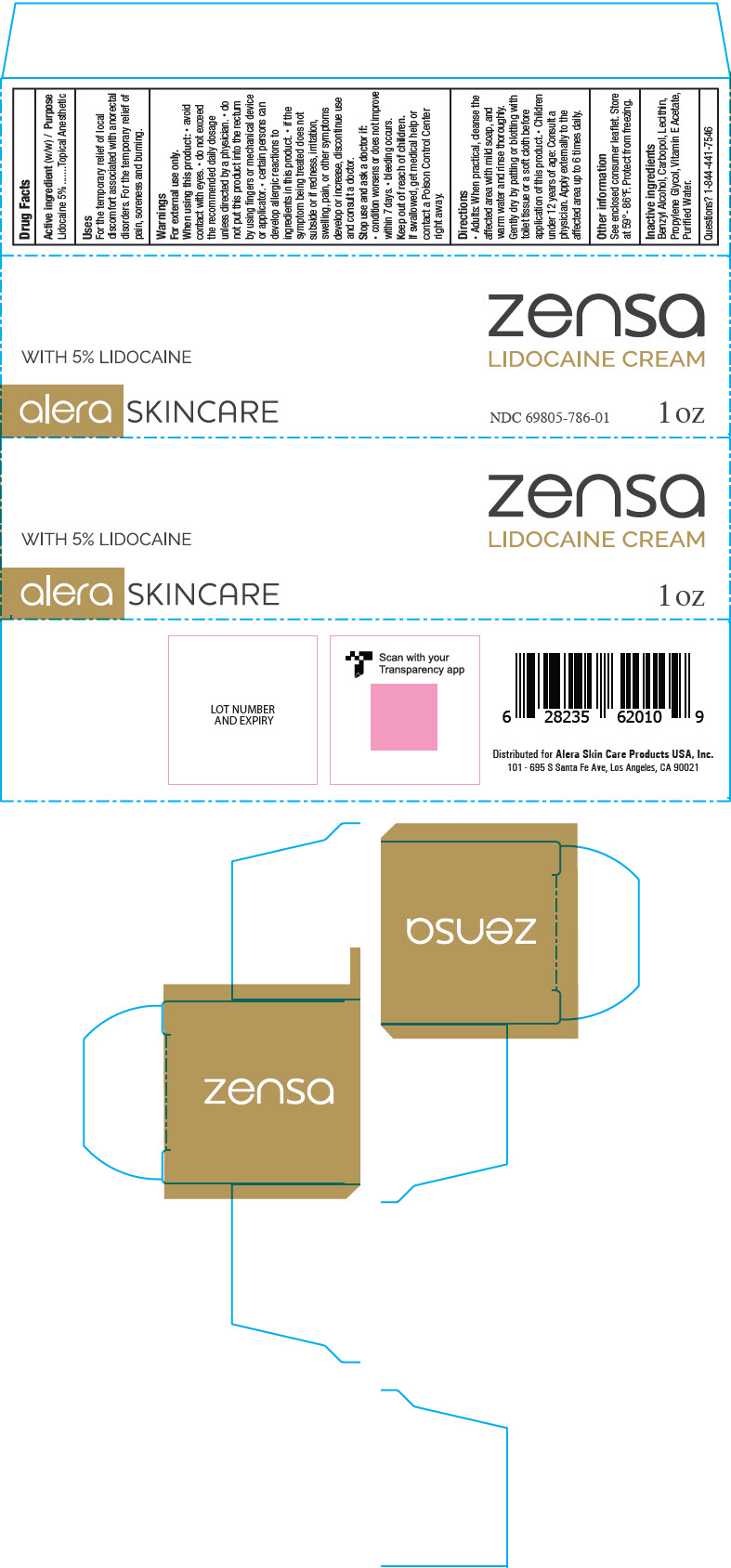
Uses
- For the temporary relief of local discomfort associated with anorectal disorders
- For the temporary relief of pain, soreness, and burning
Warnings
For external use only.
When using this product
- avoid contact with eyes
- do not exceed the recommended daily dosage unless directed by a physician
- do not put this product into the rectum by using fingers or any mechanical device or applicator
- certain persons can develop allergic reactions to ingredients in this product. If the symptom being treated does not subside or if redness, irritation, swelling, pain, or other symptoms develop or increase, discontinue use and consult a doctor
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
Directions
- Adults: when practical, cleanse the affected area with mild soap, and warm water and rinse thoroughly. Gently dry by patting or blottig with toilet tissue or a soft cloth before application of this product. Children under 12 years of age: consult a physician.
- Apply externally to the affected area up to 6 times daily